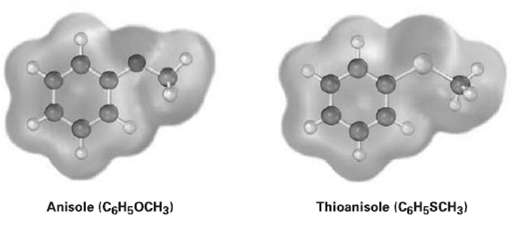
Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid,
Question:
Electrostatic potential maps of anisole and thioanisole are shown. Which do you think is the stronger acid, p-methoxybenzoic acid or p-(methylthio) benzoic acid?Explain.
Transcribed Image Text:
Anisole (CGH5OCH3) Thioanisole (C6H5SCH3)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
The electrostatic potential maps show that the aromatic ring of anisole ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which do you think is more important for organizations: downward communication or upward communication?
-
Which do you think is more important for organizations: formal or informal communication?
-
Which do you think are more important for an international marketer who is introducing a new product, a push or a pull strategy? Why? What specific types of tactics do you think are most important in...
-
You name the catastrophe, and JIT has been through it and survived. Toyota Motor Corporation has had its world-renowned JIT system tested by fire. The massive fire incinerated the main source of...
-
Jonas and Rande are facing a difficult situation. What sources of conflict contributed to the problems they are having?
-
Calculating the standard error of the mean Given s = 7.50 and N = 225, calculate s M .
-
Consider the tactics used to achieve each objective. Analyse their appropriateness, their relative strengths and weaknesses. Would you do anything differently?
-
Ron Sargeant's electric bill from the Longwood Power Authority is shown. If his meter reading for December 17 is 52,344, find the total charges for the December bill. Include all rates and charges as...
-
Consumer Decision Making Please provide an example of how you went through all stages of a consumer decision making process and detail the influences that you encountered. Try to pick a high-cost,...
-
News.com is a Web site that offers users access to current national and international news stories. News.com does not charge users a fee for accessing the site, but rather charges advertisers $ 0.05...
-
The following carboxylic acid can?t be prepared from an alkyl halide by either the nitrile hydrolysis route or the Grignard carboxylation route. Explain.
-
Give IUPAC name for the followingcompounds: (c) NC. CH 2 () (a) CHCH2CH2CH CH2 CH () CH CH2CO2H (d) (e) CHH2H2H2CH3 CHCN CH (g) Br (h) CN BRCH2CHCH2CH2CO2H
-
You want to prepare 500.0 mL of 1.000 M KNO3 at 20C, but the lab (and water) temperature is 24C at the time of preparation. How many grams of solid KNO3 (density 2.109 g/mL) should be dissolved in a...
-
9. [10] Suppose that B and W are BMs and that they are correlated with correlation coefficient P (-1, 1) in the sense that the correlation coefficient between Bt and Wt for all t>0. Then we can...
-
You have just incorporated and started your business. Your corporate pre-tax profit is $40,000. This is your only source of income. This income is eligible for the Small Business Deduction and is...
-
4. Provide the information requested in the statements below: a) Find and draw all C's that do not contain H's (if any). For this, redraw the structure where you show the d ('s). N b) Find and draw...
-
Suppose that f(x) = 8x + 5. (A) Find the slope of the line tangent to f(x) at x = 7. (B) Find the instantaneous rate of change of f(x) at x = -7. C) Find the equation of the line tangent to f(x) at x...
-
Whichof the following regarding the relationship between business risk and financial risk is least accurate based on our discussions in class? A. Business risk represents uncertainty caused by...
-
IPO Underpricing The Woods Co. and the Garcia Co. have both announced IPOs at $40 per share. One of these is undervalued by $9, and the other is overvalued by $4, but you have no way of knowing which...
-
The graph of the sequence whose general term is an = n - 1 is which of the following? [8.1] A. B. TITTT 3-2-1 23.45 2.3.4
-
One kind of polyester is a condensation copolymer formed from terephthalic acid and ethylene glycol. Draw the structure of the dimer. HO Terephthalic acid OH HD-CH-CH-OH Ethylene glycol
-
Direct oxidation of an aldose affects the aldehyde group first, converting it to a carboxylic acid, and most oxidizing agents that will attack 1o alcohol groups will also attack 2o alcohol groups....
-
Write three-dimensional formulas for each aldotetrose and ketopentose isomer in Practice Problem 22.1 and designate each as a d or l sugar.
-
Give appropriate structural formulas to illustrate each of the following: (a) An aldopentose (b) A ketohexose (c) An l-monosaccharide (d) A glycoside (e) An aldonic acid (f) An aldaric acid (g) An...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


