One kind of polyester is a condensation copolymer formed from terephthalic acid and ethylene glycol. Draw the
Question:
One kind of polyester is a condensation copolymer formed from terephthalic acid and ethylene glycol. Draw the structure of the dimer.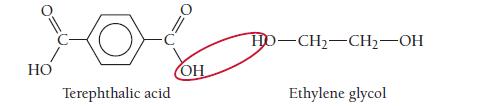
Transcribed Image Text:
HO Terephthalic acid OH HD-CH₂-CH₂-OH Ethylene glycol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The polyester named Lactomer® is an alternating copolymer of lactic acid and glycolic acid. Lactomer is used for absorbable suture material because stitches of Lactomer hydrolyze slowly over a...
-
Glyptal resin makes a strong, solid polymer matrix for electronic parts. Glyptal is made from terephthalic acid and glycerol. Draw the structure of Glyptal, and explain its remarkable strength and...
-
Ethylene glycol is used as an automobile antifreeze and in the manufacture of polyester fibers. The name glycol stems from the sweet taste of this poisonous compound. Combustion of 6.38 mg of...
-
Both high-income and low-income employees are covered by cafeteria plans. Under such plans, all employees may select from a list of non-taxable fringe benefits or they may elect to receive cash in...
-
On December 23, Wyman, a lawyer representing First National Bank & Trust (defendant), wrote to Zeller (plaintiff) stating that he had been instructed to offer a building to Zeller for sale at a price...
-
Bring out the various factors which influence the selection process.
-
Briefly describe why casino resorts are superior to stand-alone operations.
-
Data for Kurtzel Company are presented in P12-7A. Further analysis reveals the following. 1. Accounts payable pertain to merchandise suppliers. 2. All operating expenses except for depreciation were...
-
30, $147,000 Asume the company had a loss (instead of the gain) on the sale of equipment of $3,000, determine IBT Use the following to answer questions 31-32 Last year T, Inc., had the following...
-
a. How much cash does Patterson have on hand relative to its total assets? b. What proportion of Patterson's assets has the firm financed using short-term debt? Long-term debt? c. What percent of...
-
Nomex, a condensation copolymer used by firefighters because of its flame-resistant properties, forms from isophthalic acid and m-aminoaniline. Draw the structure of the dimer. HO Isophthalic acid OH...
-
Saran, the polymer used to make Saran Wrap, is an addition polymer formed from two monomersvinylidene chloride and vinyl chloride. Draw the structure of the polymer. H C=C Cl H Cl Vinylidene chloride...
-
Describe graphically the average amount of money a person spends on movie tickets each month (in per month). 6.0 5.3 4.0 5.7 4.3 6.8 4.1 3.4 3.9 5.0 6.5 4.7 6.2 2.5 1.9 4.5 3.1 2.4 3.5 2.3 3.1 5.5
-
Which (if any) of the light bulbs in Figure P31.17 are connected to other bulbs in parallel? Data from Figure P31.17 (e) (e) e H (e) e E (e)
-
Follett Company makes handwoven blankets. The companys master budget appears in the first column of the table. Required: Complete the table by preparing Folletts flexible budget for 4,000, 6,000, and...
-
If assets increase by \(\$ 50\) and liabilities decrease by \(\$ \mathbf{3 0}\), stockholders' equity must: a. Remain unchanged b. Increase by \(\$ 80\) c. Decrease by \(\$ 70\) d. Decrease by \(\$...
-
Determine the slope at \(A\) of the W14 \(\times 26\) beam made from A992 steel. 8 kip A -5 ft 5 ft- B 8 kip C -5 ft 5 ft- D
-
On December 20, 2002, New Yorks attorney general, Eliot Spitzer, announced a \($1.4\) billion settlement ending a multiregulator probe of ten brokerages that alleged that investors were duped into...
-
Assign a priority order to a. -C¡CH and -CH=CH2 b. -CH=CH2 and c. -CH=O, -CH=CH2, -CH2CH3, and -CH2OH
-
Calculate I, , and a for a 0.0175 m solution of Na 3 PO 4 at 298 K. Assume complete dissociation. How confident are you that your calculated results will agree with experimental results?
-
(Challenging) Building on the concept of equipartition, demonstrate that for any energy term of the form ax 2 , where α is a constant, the contribution to the internal energy is equal...
-
For a system of energy levels, m = m 2 , where is a constant with units of energy and m = 0, 1, 2, , . What is the internal energy and heat capacity of this system in the high-temperature limit?
-
What is the contribution to the internal energy from translations for an ideal monatomic gas confined to move on a surface? What is the expected contribution from the equipartition theorem?
-
Case 9-47 Comprehensive Master Budget; Short-Term Financing; Acquisition of Robotic Equipment (LO 9-2, 9-3, 9-5, 9-6) Skip to question [The following information applies to the questions displayed...
-
Briefly compare and contrast Return on Investment with Residual Income. Be sure to discuss the advantages and disadvantages of each. When might it be more appropriate to use one method over another.
-
How is this done? The answer should be in a T-chart form in 3 different categories: Direct Materials Inventory, Work-in-Process, & Finish Goods Inventory. Rogers Company had inventories at the...
Fuzzy Statistical Decision Making Theory And Applications 1st Edition - ISBN: 3319817930 - Free Book

Study smarter with the SolutionInn App


