Explain how the amperometric end-point detector in Figure 16-8 operates. Figure 16-8 To constant-current coulometric power supply
Question:
Explain how the amperometric end-point detector in Figure 16-8 operates.
Figure 16-8
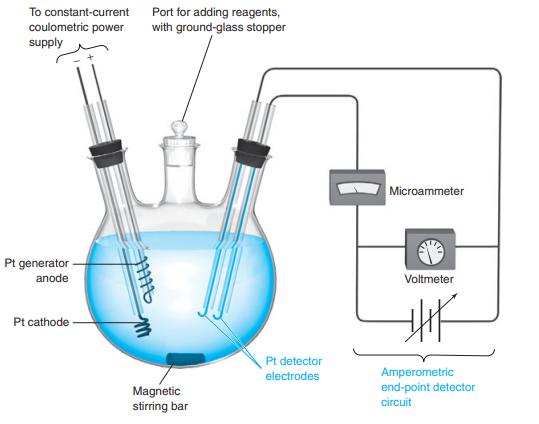
Transcribed Image Text:
To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt generator anode Voltmeter Pt cathode Pt detector Amperometric end-point detector electrodes Magnetic stirring bar circuit
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
When excess Br 2 appears in the solutio...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
(a) How does the amperometric glucose monitor in Figure 16-10 work? (b) Why is a mediator advantageous in the glucose monitor? (c) How does the coulometric glucose monitor in Figure 16-12 work? (d)...
-
Explain how the end point is detected in a Karl Fischer titration in Figure 16-32. Figure 16-32 Pt electrodes for coulometric Pt electrodes for generation of l, bipotentiometric end-point detection -...
-
Explain how replication shown in these steps from figure 6.17 would differ from those in figure 9.29. DNA DNA (3 Duplication of phage components; replication of virus genetic material 2) Penetration...
-
Show that the allocation which solves the first-order condition from Eq. (3.7) for a social optimum satisfies the second-order conditions. Equation 3.7 -C(e) = D'(E) i=1,.... (3.7)
-
Ceda Co. has equipment that cost $80,000 and that has been depreciated $50,000. Instructions Record the disposal under the following assumptions. (a) It was scrapped as having no value. (b) It was...
-
The financial statements of Apple Inc. are presented in Appendix A. Instructions Answer the following questions using the financial statements and the notes to the financial statements. a. What were...
-
Go to www.brighthubpm.com/monitoring-projects/ 51982-understanding-the-s-curve-theory-for-project-management- monitoring/ and read the article on the multiple uses of project S-curves. What does the...
-
DeQuan Matthews started a sports travel business to help college students travel to out of town sporting events. He engaged in the following activities to establish Sports to Go. 1. Deposited $10,000...
-
Average Rate of Return The following data are accumulated by Lone Peak Inc. in evaluating two competing capital investment proposals: Determine the expected average rate of return for each proposal....
-
Was Exxon derelict in its accounting for this disaster? Why or why not? What would be the impact on common debt ratios in those previous years had Exxon accounted for the liability in the past as...
-
Electroplating efficiency. Nickel was electrolytically plated onto a carbon electrode from a bath containing 290 g/L NiSO 4 6H 2 O, 30 g/L B(OH)3, and 8 g/L NaCl at - 1.2 V vs. Ag | AgCl. The most...
-
The sensitivity of a coulometer is governed by the delivery of its minimum current for its minimum time. Suppose that 5 mA can be delivered for 0.1 s. (a) How many moles of electrons are delivered by...
-
An electric baseboard heater can convert 100% of the electric energy used into heat that flows into the house. Since a gas furnace might be located in a basement and sends exhaust gases up the...
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Oscillations and Resonance Name Lab Procedure Answer questions in red. Download and run the HTMLS application \"resonance\". Driving force: 30 N Driving equency: 5 rad. '5 Spring constant: 5 - 'Irn...
-
What is the purpose of zoning regulations?
-
What is the back work ratio? What are typical back work ratio values for gas-turbine engines?
-
Solubility products predict that cation A 3+ can be 99.999% separated from cation B 2+ by precipitation with anion X - . When the separation is tried, we find 0.2% contamination of AX 3 (s) with B 2+...
-
Identify the Lewis acids in the following reactions: a. b. BF3 + NH3 FB-NH3
-
Gaseous SO 2 is created by combustion of sulfur-containing fuels, especially coal. Explain how O 2 in the atmosphere makes acidic rain.
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...
-
5. The current spot exchange rate is 0.95/$ and the three-month forward rate is 0.91/$. Based on your analysis of the exchange rate, you are pretty confident that the spot exchange rate will be...

Study smarter with the SolutionInn App


