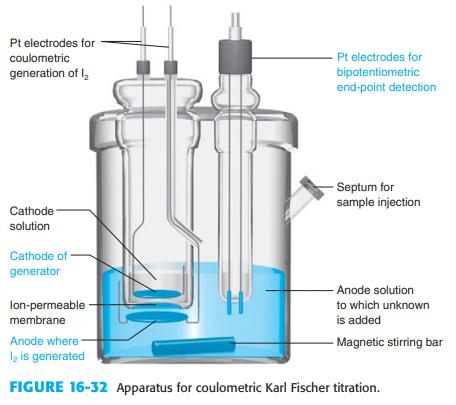
Explain how the end point is detected in a Karl Fischer titration in Figure 16-32. Figure 16-32
Question:
Explain how the end point is detected in a Karl Fischer titration in Figure 16-32.
Figure 16-32
Transcribed Image Text:
Pt electrodes for coulometric Pt electrodes for generation of l, bipotentiometric end-point detection - Septum for sample injection Cathode solution Cathode of generator - Anode solution lon-permeable - to which unknown membrane is added Anode where l2 is generated - Magnetic stirring bar FIGURE 16-32 Apparatus for coulometric Karl Fischer titration.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
The bipotentiometric detector maintains a constant current ...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Write the chemical reactions that show that 1 mol of I 2 is required for 1 mol of H 2 O in a Karl Fischer titration.
-
Explain how the amperometric end-point detector in Figure 16-8 operates. Figure 16-8 To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt...
-
Explain how a molecule is assigned to a point group.
-
Write a recursive program to draw plasma clouds, using the method suggested in the text.
-
Lofton Company purchased a delivery truck. The total cash payment was $27,900, including the following items. Negotiated purchase price .......... $24,000 Installation of special shelving ..........
-
What are the two fundamental approaches by which ethical business reasoning has traditionally been characterized?
-
Search the Internet for links to the Boston Tunnel, The Big Dig; the Channel Tunnel, The Chunnel; and Londons Millennium Dome. Why do you think these projects were supported to their conclusion in...
-
Storm, Inc. purchased the following available-for-sale securities during 2014, its first year of operations: The market price per share for the available-for-sale security portfolio on December 31,...
-
B. Paku Bhd acquired a 70% interest in Oste Bhd on June 2015 for RM35,500,000. On this date, the ordinary shares of Oste Bhd was RM35,000,000 and the retained profit was RM10,000,000 On 1 January...
-
What attractive features are offered by counterblow forging equipment, or impactors?
-
What is the purpose of the Nafion membrane in Figure 16-30? Figure 16-30 40
-
Consider the following electrolysis reactions. (a) Calculate the voltage needed to drive the net reaction if current is negligible. (b) Suppose that the cell has a resistance of 2.0 and a current of...
-
Austin Grocers recently reported the following 2008 income statement (in millions of dollars): Sales $700 Operating costs including depreciation 500 EBIT $200 Interest 40 EBT $160 Taxes (40%) 64 Net...
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
What might happen if you failed to have the necessary contractors license and an owner failed to pay you?
-
A woman at a point A on the shore of a circular lake with radius 2 mi wants to arrive at the point C diametrically opposite on the other side of the lake in the shortest possible A time. She can walk...
-
Using activities, calculate the pH and concentration of H + in 0.050 M LiBr at 25 C.
-
Using activities, calculate the pH and concentration of H + in 0.050 M LiBr at 25C.
-
A 40.0-mL solution of 0.040 0 M Hg 2 (NO 3 ) 2 was titrated with 60.0 mL of 0.100 M KI to precipitate Hg 2 I 2 (K sp = 4.6 10 -29 ). (a) Show that 32.0 mL of KI are needed to reach the equivalence...
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


