Explain which compound has a faster rate of SN1 reaction. a) c) HC CI CI or or
Question:
Explain which compound has a faster rate of SN1 reaction.
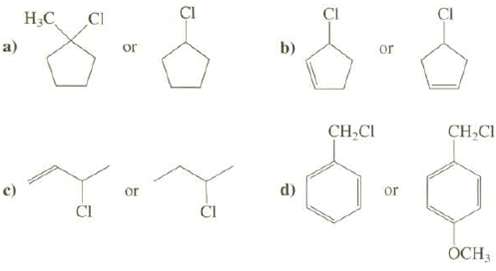
Transcribed Image Text:
a) c) H₂C CI CI or or J d) CI CH₂CI or or D CH₂Cl OCH 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
For the S N 1 reaction formation of the carbocation is the rate limitin...View the full answer

Answered By

Shaira grace
I have experience of more than ten years in handing academic tasks and assisting students to handle academic challenges. My level of education and expertise allows me communicate eloquently with clients and therefore understanding their nature and solving it successfully.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain which compound has a faster rate of reaction withHCI: b) or or NO2 or
-
Explain which compound has a faster rate of SN2 reaction: a) HC CI CI c) PhCHCH3 or H CI Cl or CHCHCH3 b) CI CH3 CH3 or U
-
Which of these compounds would have faster rate of SN1 reaction? a) b) c) d) e) CI ta Ph Ph -CI or Br or Br or or -Cl or + CH3 Ph -Cl Br Br ta -CI
-
What other types of contingency planning should Matt and Chris include to make the report comprehensive? Please explain the relevance of each suggestion.
-
Describe the problems that can arise in using process costing to estimate the cost of the services produced in mass service entities.
-
Explain the differences between how inventory purchases are recorded in a periodic system and in a perpetual system. Also explain the differences in recording sales between a periodic system and a...
-
Use the WBS you developed in Task 3 to begin creating a Gantt chart using your choice of software. Do not enter any durations or dependencies. Print the resulting Gantt chart on one page, and be sure...
-
James Equipment Company sells computers for $1,500 each and also gives each customer a 2-year warranty that requires the company to perform periodic services and to replace defective parts. During...
-
1) how does the Fed prevent a potential financial crisis?, do you agree with their methods?(explain your reasoning) 2) what are the monetary policies the Fed can apply?, which would be more...
-
Client's Facts: The client found a check written out to cash in the amount of $750. The check was completely made out when he found it. He took it to the bank, signed it on the back as instructed by...
-
Consider the free energy versus reaction progress diagram for the SN2 reaction shown in Figure 8.1. Does the transition state for this reaction have the C Cl bond less than half broke, approximately...
-
Arrange these compounds in order of decreasing SN1 reaction rate. Ph CI CI CI Ph CI Ph
-
ADM, Inc., an electronics manufacturer, uses growth in earnings per share (EPS) as a guideline for evaluating executive performance. ADM executives receive a bonus of $5,000 for every penny increase...
-
Use your own academic report, issued by your institute, as an example. Ask a database administrator how they use normalization steps to transform the details of the report into a set of relations in...
-
Francis Corp. has two divisions, Eastern and Western. The following information for the past year is for each division: Francis has established a hurdle rate of 9 percent. Required: 1. Compute each...
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Use values of r cov (Table 17.1) to estimate the XY bond lengths of ClF, BrF, BrCl, ICl and IBr. Compare the answers with values in Fig. 17.8 and Table 17.3, and comment on the validity of the method...
-
From a square whose side has length \(x\), measured in meters, create a new square whose side is \(10 \mathrm{~m}\) longer. Find an expression for the sum of the areas of the two squares as a...
-
Based on what you know now, what are your A-item priorities? Given these priorities, what do you need to do during your transition to lay the necessary groundwork for achieving them? AppendixLO1
-
The MIT Sloan School of Management is one of the leading business schools in the U.S. The following table contains the tuition data for the masters program in the Sloan School of Management. a. Use...
-
Find the slope of the line through each pair of points. (8,9), (8, 16)
-
How would you prepare ds-2-butene starting from propyne, an alkyl halide, and any other reagents needed? This problem cant be worked in a single step. Youll have to carry out more than one reaction.
-
Beginning with 4-octyne as your only source of carbon, and using any inorganic reagents necessary, how would you synthesize the following compounds? (a) cis-4-Octene (b) Butanal (c) 4-Bromooctane (d)...
-
Beginning with acetylene and any alkyl halides needed, how would you synthesize the following compounds? (a) Decane (b) 2, 2-Dimethylhexane (c) Hexanal (d) 2-Heptanone
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


