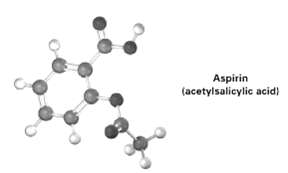
Following is a molecule model of aspirin (acetylsalicylic acid). Identify the hydribization of each carbon atom in
Question:
Following is a molecule model of aspirin (acetylsalicylic acid). Identify the hydribization of each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (gray = c. red = O, ivory =H).
Transcribed Image Text:
Aspirin (acetylsalicylic acid)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
010 IU CIH v0 O O 6 sp Aspirin ...View the full answer

Answered By

Larlyu mosoti
I am a professional writer willing to do several tasks free from plagiarism, grammatical errors and submit them in time. I love to do academic writing and client satisfaction is my priority. I am skilled in writing formats APA, MLA, Chicago, and Harvard I am a statistics scientist and I can help out in analyzing your data. I am okay with SPSS, EVIEWS, MS excel, and STATA data analyzing tools.
Statistical techniques: I can do linear regression, time series analysis, logistic regression, and some basic statistical calculations like probability distributions. . I'm ready for your working projects!
Services I would offer:
• Academic writing.
• Article writing.
• Data entry.
• PDF conversion.
• Word conversion
• Proofreading.
• Rewriting.
• Data analyzing.
The best reason to hire me:
- Professional and Unique work in writing.
- 100% satisfaction Guaranteed
- within required time Express delivery
- My work is plagiarism Free
- Great communication
My passion is to write vibrantly with dedication. I am loyal and confident to give my support to every client. Because Client satisfaction is much more important to me than the payment amount. A healthy client-contractor relationship benefits in the longer term. Simply inbox me if you want clean work.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What is the hydribization of each carbon atom in acetonitrile (Problem 1.25)?
-
Tell the number of hydrogens bonded to each carbon atom in the following substances and give the molecular formula ofeach: OH H (a) (b) CO2CH3 Ephedrine Cocaine
-
Describe the hybrid orbitals used by each carbon atom in the following molecules: a. b. C-C-C-OH
-
Like Death Valley, the Dead Sea is a place where you can walk on dry land below sea level, and an apparently unmotivated hole in the ground along a strike-slip fault. How do you think it formed?
-
Determine the percentage of vesting for the following employees. See Exhibits 19.1 and 19.2. a. Glenda has five years of service completed as of September 23, 2016, her employment anniversary date....
-
Orrin DAmato, single, participates in his firms pension retirement plan. This year, his modified adjusted gross income will be about $75,000. How much of his compensation may DAmato contribute to an...
-
Are present channels of distribution reliable and cost effective?
-
Liquid water is fed to a boiler at 24C and 10bar and is converted at constant pressure to saturated steam. Use the steam tables to calculate H (kJ/kg) for this process, and then calculate the heat...
-
Bond premium, entries for bonds payable transactions Rodgers Corporation produces and sells football equipment. On July 1,20Y1, Rodgers issued $20,700,000 of 10 -year, 13% bonds at a market...
-
One Trick Pony (OTP) incorporated and began operations near the end of the year, resulting in the following post-closing balances at December 31: The following information is relevant to the first...
-
Draw a line-bond structure for 1, 3-butadiene, H2C = CH CH = CH2; indicate the hydribization of each carbon; and predict the value of each bond angle.
-
Draw a line-bond structure for the propyne, CH3C CH; indicate the hydribization of each carbon; and predict a value for each bond angle.
-
What are the six goals the Fed is required by government legislation to achieve? Which two goals are the most important? Why?
-
The accountant at EZ Toys, Inc. is analyzing the production and cost data for its Trucks Division. For October, the actual results and the master budget data are presented below. Actual Results:...
-
2. 2D Design (4 points): The Pawnee Department of Parks and Recreation has received alarming reports that their picnic tables might be unstable. Examine the picnic table design below (which weighs 50...
-
Answer 3-10 Cash flow Bailey Corporations income statement (dollars are in thousands) is given here: Sales Operating costs excluding depreciation $14,000,000 and amortization EBITDA Depreciation and...
-
You want to create a database for computer lab management. You want to keep track of the following information (Type your answer): The information about computer/workstation such as station ID,...
-
You have been hired for a newly created position for a large medical office that employs five MDs and four Advanced Practice Registered Nurses (APRNs). Upper leadership created this position due to...
-
Various summations of daily and weekly reports. LO.1
-
Solve each equation or inequality. |6x8-4 = 0
-
a. In vapor-liquid equilibrium, mixtures sometimes occur in which the compositions of the coexisting vapor and liquid phases are the same. Such mixtures are called azeotropes. Show that a binary...
-
On the basis of the rule that anything unusual about a structure must be shown explicitly, the nitrogen in the structure NH 3 is seen to have an unshared pair of electrons whether these electrons are...
-
Ozone O 3 is a form of oxygen found in the upper atmosphere. It has the connectivity O OO and is neutral. (a) Show a Lewis structure for ozone. (b) Calculate the formal charge on each oxygen of...
-
(a) Draw a Lewis structure for the carbonate anion CO 3 2 each oxygen is bonded only to the carbon. (b) Calculate the formal charge on each atom. (c) What is the shape of this species? (d)...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


