Tell the number of hydrogens bonded to each carbon atom in the following substances and give the
Question:
Tell the number of hydrogens bonded to each carbon atom in the following substances and give the molecular formula ofeach:
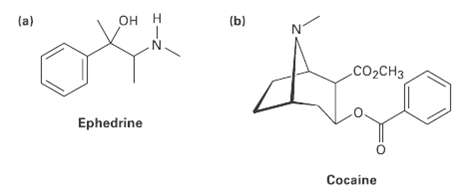
Transcribed Image Text:
OH H (a) (b) CO2CH3 Ephedrine Cocaine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (21 reviews)
2 2H 3H CH3 2 H 3H COCH...View the full answer

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Tell how much hydrogen is bonded to each carbon in the following compounds, and give the molecular formula of each substance: (b) NHCH3 Adrenaline Estrone (a hormone)
-
Star (*) each asymmetric carbon atom in the following examples, and determine whether it has the (R) or (S) configuration. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH H CH,CH, H,C CH,CH, H H CH CCH, CI H...
-
Indicate the molecular geometry around each carbon atom in the compound H;CCH=CHCH,CCH,COOH
-
In what circumstances have courts opted to use the substantial-factor test rather than the but-for test?
-
John Benson, age 40, is single. His Social Security number is 111-11-1111, and he resides at 150 Highway 51, Tangipahoa, LA 70465. John has a 5-year-old child, Kendra, who lives with her mother,...
-
Use a numerical solver and Eulers method to obtain a four-decimal approximation of the indicated value. First use h = 0.1 and then use h = 0.05. y' = x 2 + y 2 , y(0) = 1; y(0.5)
-
Among the ANES unique group of people who simultaneously rate Christian Fundamentalists and atheists at 100 on the feeling thermometer, is there a difference between men and women with regard to how...
-
Suppose your cookie company is now a corporation that has granted franchises to more than 50 stores. Currently, only 10 of the 50 stores have computerized machines for mixing cookie dough. Because of...
-
1 ) If the profit on a trick ski is $ 5 0 and the profit on a slalom ski is $ 3 0 , how many of each type of ski should be manufactured each day to realize a maximum profit? What is the maximum...
-
Create an app with three Button elements labeled Text One, Text Two, and Text Three. When any of these Button elements are clicked, launch a second Activity. That second Activity should contain a...
-
The following molecular models are representations of (a) Adenine and (b) Cytosine constituents of DNA. Indicate the positions of the multiple bonds and lone pairs for both, and draw skeletal...
-
Identify the most electronegative element in each of the following molecules: (a) CH2FC1 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li
-
A 30-year $140,000 mortgage has a rate of 8 percent. What are the interest and principal portions in the first payment? In the second?
-
Listed below are the lead concentrations (in ug/g) measured in different Ayurveda medicines. Ayurveda is a traditional medical system commonly used in India. The lead concentrations listed here are...
-
The assignment states to use a movie and talk about 2 scenes where physics ideas are used. The rubric is shown and 6 big ideas that can be talked about are also attached. Background Information...
-
A series of computer and backup system failures caused the loss of most of the company records at Stotter, Incorporated. Information technology consultants for the company could recover only a few...
-
Future value of an annuity Using the values below, answer the questions that follow. (Click on the icon here in order to copy the contents of the data table below into a spreadsheet.) Deposit period...
-
Mercury, Incorporated, produces cell phones at its plant in Texas. In recent years, the company's market share has been eroded by stiff competition from overseas. Price and product quality are the...
-
The driving forces push for change so that the organization advances to a better position. The resisting forces on the other hand, maintain an equilibrium by negating the power of these driving...
-
What is a lobbyist in US? How did this term emerge?
-
Nitrogen at 1 atm and 25C is to be compressed to 5 bar in an adiabatic, isentropic compressor. What is the temperature of the nitrogen stream exiting the compressor? If the flowrate of nitrogen is...
-
Explain which compound has the higher solubility in water.
-
Which of these compounds exhibit cis-trans isomerism? Draw both cis-trans isomers when they exist? a) CHCHCH=CHCH CH3 c) CHC=CHCH b) CH3CHCH=CH CI d) CHC=CHCHCH3
-
Draw the cis-trans isomers for these compounds and explain which is more stable: CH3 a) CH CH CHCOH NH c) e CH, CH2NHCH CH f CH3CH CH CH
-
Mediocre Company has sales of $120,000, fixed expenses of $24,000, and a net income of $12,000. If sales rose 10%, the new net income would be: Question 18 options: $16,800 $36,000 $13,200 $15,600
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...

Study smarter with the SolutionInn App


