For each of the metals listed in the table, compute the Pilling???Bedworth ratio. Also, on the basis
Question:
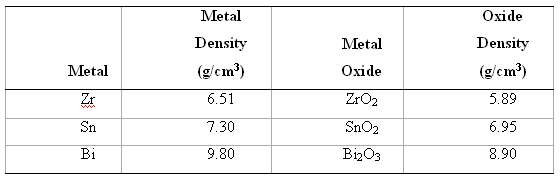
For each of the metals listed in the table, compute the Pilling???Bedworth ratio. Also, on the basis of this value, specify whether or not you would expect the oxide scale that forms on the surface to be protective, and then justify your decision. Density data for both the metal and its oxide are alsotabulated.
Transcribed Image Text:
Oxide Metal Metal Density (g/em?) Density Metal Oxide (g/cm?) 5.89 6.51 ZrO2 SnO2 Zr Sn 6.95 7.30 Bi 8.90 BizO3 9.80
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
With this problem we are given for three metals their densities oxide chemical formulas ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
For each of the metals listed in the following table, compute the Pilling-Bedworth ratio. Also, on the basis of this value, specify whether you would expect the oxide scale that forms on the surface...
-
For each of the following, tell whether or not you would expect it to have a strong seasonal component and why. a. Sales of colorful wrapping paper, recorded monthly. b. The number of air travelers...
-
For each of the following compounds, determine whether or not you would expect its IR spectrum to exhibit a signal to the left of 3000 cm -1 a. b. c. . d. e. f.
-
A common stock pays an annual dividend that increases by 4% annually and sells for $35 per share. If the market rate of return on this stock is 8%. What is the amount of the ?last dividend paid $1.53...
-
Explain the connection between a firm's accounting-based profitability and its cash cycle?
-
The following questions concern quality control standards. Choose the best response.a. The nature and extent of a public accounting firm?s quality control policies and procedures depend on: b. Which...
-
What impact does the availability of funds have on management's evEiluation of capital investment opportunities? LO.1
-
One of the biggest problems of student writers is paraphrasing secondary sources correctly to avoid plagiarism. Your Task. For each of the following, read the original passage. Analyze the...
-
A company reports the following information for the single product it manufactures
-
Complete Form 941 for the 4th quarter for TCLH Industries (which is located at 202 Whitmore Avenue, Durham, NC 27701; Employer Identification #44-4444444). Assume that all necessary deposits were...
-
Briefly describe the two techniques that are used for galvanic protection.
-
According to Table 17.3, the oxide coating that forms on silver should be nonprotective, and yet Ag does not oxidize appreciably at room temperature and in air. How do you explain this...
-
The magnetic field component of a plane wave in a lossless dielectric [is H = 30 sin (2 X 10 8 t 5x) a z mA/m (a) If r = l. find r .. (b) Calculate the wavelength and wave velocity. (c) Determine...
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
25 of 27 > This test: 96 point(s) possible This question: 3 point(s) possible Submit test Identical twins come from a single egg that split into two embryos, and fraternal twins are from separate...
-
Use vertical analysis to compare finan cial statement items with each other and with industry averages. AppendixLO1
-
What is the amount of total interest dollars earned on a $5,000 deposit earning 6% for 20 years?
-
Create a query that summarizes the value of products currently in inventory. Note that the value of each product is a result of multiplying the units currently in inventory by the unit price. Sort...
-
Why is it undesirable to minimize friction between the work-piece and tooling in a rolling operation?
-
Why is it important to control the finishing temperature of a hot-rolling operation?
-
Discuss the relative advantages and typical uses of two-high rolling mills with large-diameter rolls, three-high mills, and four-high mills.
-
A project will generate annual cash flows of $237,600 for each of the next three years, and a cash flow of $274,800 during the fourth year. The initial cost of the project is $749,600. What is the...
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost

Study smarter with the SolutionInn App


