From the two standard additions of 50 pm Fe(III) in Figure 16-23, find the concentration of Fe(III)
Question:
From the two standard additions of 50 pm Fe(III) in Figure 16-23, find the concentration of Fe(III) in the seawater. Estimate where the baseline should be drawn for each trace and measure the peak height from the baseline. Consider the volume to be constant for all three solutions.
Figure 16-23
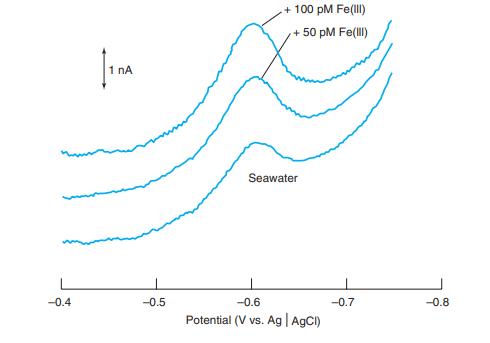
Transcribed Image Text:
+ 100 pM Fe(ll) +50 pM Fe(lI) 1 nA Seawater --0.4 -0.5 -0.6 --0.7 -0.8 Potential (V vs. Ag AgCI)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Cells B13 and B18 of the spreadsheet tell us that F...View the full answer

Answered By

Akshay Agarwal
I am a Post-Graduate with a specialization in Finance. I have been working in the Consulting industry for the past 8 years with a focus on the Corporate and Investment Banking domain. Additionally, I have been involved in supporting student across the globe in their academic assignments and always strive to provide high quality support in a timely manner. My notable achievements in the academic field includes serving more than 10,000 clients across geographies on various courses including Accountancy, Finance, Management among other subjects. I always strive to serve my clients in the best possible way ensuring high quality and well explained solutions, which ensures high grades for the students along-with ensuring complete understanding of the subject matter for them. Further, I also believe in making myself available to the students for any follow-ups and ensures complete support and cooperation throughout the project cycle. My passion in the academic field coupled with my educational qualification and industry experience has proved to be instrumental in my success and has helped me stand out of the rest. Looking forward to have a fruitful experience and a cordial working relationship.
5.00+
179+ Reviews
294+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The figure below shows a series of standard additions of Cu 2+ to acidified tap water measured by anodic stripping voltammetry at an iridium electrode. The unknown and all standard additions were...
-
Standard addition. A particular CO2 compound electrode like the one in Figure 14-28 obeys the equation E = constant - [RT (ln 10)/2F] log[CO2], where R is the gas constant, T is temperature (303.15...
-
The method of standard additions was used to determine nitrite in a soil sample. A 1.00-mL portion of the sample was mixed with 24.00 mL of a colorimetric reagent, and the nitrite was converted to a...
-
A space-filling curve is a continuous curve in the unit square that passes through every point. Write a recursive Turt le client that produces these recursive patterns, which approach a space-filling...
-
Benedict Company incurred the following costs. 1. Sales tax on factory machinery purchased ........... $ 5,000 2. Painting of and lettering on truck immediately upon purchase ... 700 3. Installation...
-
Before a trial begins, how can the parties obtain information and collect evidence about the case?
-
What are some of the reasons why objective project evaluation may be difficult to achieve?
-
Rothschild Chair Company, Inc., was indebted to First Lincoln Bank under a $20 million, 10% unsecured note. The note was signed January 1, 2005, and was due December 31, 2014. Annual interest was...
-
Hornsby has a single production department, and uses a process-costing system. The balance in its Work-in-Process account on January 1 was $75,000. The account was charged with direct materials,...
-
A university found that 20% of its students withdraw without completing the introductory statistics course. Assume that 20 students registered for the course. a. Compute the probability that 2 or...
-
The standard free energy change for the formation of H 2 (g) + O 2 (g) from H 2 O is G = + 237.13 kJ. The reactions are Calculate the standard voltage (E) needed to decompose water into its elements...
-
Peak current (Ip) and scan rate (I p ) are listed for cyclic voltammetry (Fe(II) Fe(III)) of a water-soluble ferrocene derivative in 0.1 M NaCl. If a graph of I p versus v gives a straight line,...
-
Poor Sarah Jane not only had a short-lived marriage but found out that her supposedly $45,000 ring was worth only half that amount. It seems that the jeweler had misrepresented it to her now...
-
If f ( x ) = - 6 x ^ 2 sin ( 3 x ) + 4 x cos ( 3 x ) what if f ' ( x ) ?
-
What is deformation?
-
What is a switched capacitor circuit?How do we design a switched capacitor circuit?
-
What is meant by wavelet fault diagnosis of an induction motor?
-
How do we design an electromagnetic relay?
-
You are a contractor with a cost plus a fee contract and the project ends up costing $50,000 more than the budget. What is the impact on you?
-
Write a paper about medication error system 2016.
-
In the series SiF4, PF3, and SF2, estimate the F-X-F bond angle in each case and explain your rationale.
-
(a) If the valence atomic orbitals of an atom are sp hybridized, how many unhybridized p orbitals remain in the valence shell? How many bonds can the atom form? (b) Imagine that you could hold two...
-
(a) Draw Lewis structures for ethane (C2H6), ethylene (C2H4), and acetylene (C2H2). (b) What is the hybridization of the carbon atoms in each molecule? (c) Predict which molecules, if any, are...
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


