Give an explanation for each of the following facts. (a) Cis- and trans-1.3-dimethylpyrrolidine rapidly interconvert. (b) The
Question:
(a) Cis- and trans-1.3-dimethylpyrrolidine rapidly interconvert.
(b) The following compound exists as the enamine isomer shown rather than as an imine:
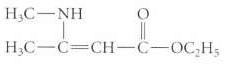
Transcribed Image Text:
HAC-C-CH-C-OCH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
a Cisand trans13dimethylpyrrolidine rapidly interconvert because a...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Provide an explanation for each of the following phenomena: (a) Solid argon (m.p. -189.2°C; b.p. -185.7°C) can be prepared by immersing a flask containing argon gas in liquid nitrogen (b.p....
-
Offer an explanation for each of the following observations. The racemate of 2,2,5,5 -tetramethyl-3,4-hexanediol existst with a strong intramolecular hydrogen bond, but the meso stereoisomer has no...
-
Offer an explanation for each of the following observations, including the structure of following product and the role of the quaternary ammonium salt. When sodium benzenethiolate, Na+ PhS- , is...
-
The following data have been extracted from the financial statements of Prentiss, Inc., a calendar-year merchandising corporation: Total sales for 2018 were $1,200,000 and for 2017 were $1,100,000....
-
Which design typemechanistic or organicbest fits Starbucks and why?
-
Compare and contrast the positive and negative aspects of both anger and empathy as common emotions that people may display at work.
-
Explain why functional currency should be remeasured, rather than translated, when a foreign entitys functional currency is highly inflationary. AppendixLO1
-
The Ombudsman Foundation is a private not-for-profit organization providing training in dispute resolution and conflict management. The Foundation had the following pre-closing trial balance at...
-
A property was purchased for $8439.00 down and payments of $1327.00 at the end of every year for 6 years. Interest is 12% per annum compounded quarterly. What was the purchase price of the property?...
-
On 1 January 20x6, Company X acquires the entire share capital of $500,000 comprising of 500,000 ordinary shares in Company Y. Retained earnings as that date amounted to $100,000. In previous years,...
-
When p-aminophenol reacts with one molar equivalent of acetic anhydride, a compound acetaminophen (A, C8H9NO2) is formed that dissolves in dilute NaOH. When A is treated with one equivalent of NaOH...
-
Show how the insecticide cctrban'l can be prepared from rnethyl isocyanate, O-C-NHCH3 or carbaryl
-
Dolphin Ceramics produces large planters to be used in urban landscaping projects. A special earth clay is used to make the planters. The standard quantity of clay used for each planter is 24 pounds....
-
7. A psychiatrist is testing a new ADHD Medication, which seems to have the potentially harmful side effect of increasing the heart rate. For a sample of 50 clinical study participants whose pulse...
-
Determine the type of engagement that your colleague completed for the client. Justify the selected engagement type for the client. Assess the purpose of each financial statement for the client's...
-
Mills Corporation acquired as a long-term investment $235 million of 8% bonds, dated July 1, on July 1, 2024. Company management has classified the bonds as an available-for-sale investment. The...
-
A force of 28 pounds acts on the pipe wrench shown in the figure below. 18 in. 30 (a) Find the magnitude of the moment about O by evaluating ||OA x F||. (0 0 180) Use a graphing utility to graph the...
-
Module 1 1. There has been a rise in cases of measles in RI. The RI Health Department is wondering if the rate of MMR vaccinations has declined since the start of the COVID-19 pandemic. The...
-
Assume you wish to evaluate the performance of a portfolio that had a 14 percent average return for a period of time, with a standard deviation of 23 percent. The market for the same period had an...
-
In the circuit shown in Figure 4, a battery supplies a constant voltage of 40 V, the inductance is 2 H, the resistance is 10, and l(0) = 0. (a) Find l(t). (b) Find the current after 0.1s.
-
Propose skeletal structures for compounds that satisfy the following molecular formulas. There is more than one possibility in each case. (a) C5H12 (b) C2H7N (c) C3H6O (d) C4H9Cl
-
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds. And draw a skeletal...
-
Convert each of the following molecular models into skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray = C, red ?...
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


