Give IUPAC names (or the following structures: (a) (b) (c) -- CH- H (d) (e)
Question:
Give IUPAC names (or the following structures:
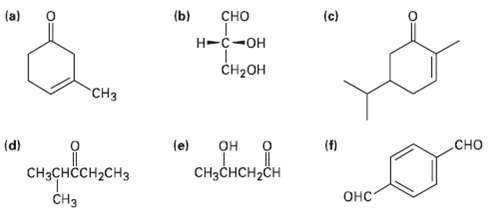
Transcribed Image Text:
(a) (b) сно (c) Н-с-он CH-он "СHз сно (d) (e) (f) он CнзснсH-CH CHзснссH2сHз Онс" Cнз O:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (18 reviews)
a CH3 3Methyl3cyclo hexenone d b CHCHCH2CH ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the IUPAC names of the following alkanes. (a) CH3C(CH3)2CH(CH)CH3)CH2CH2CH(CH3)2 (b) (c) (d) (e) (f) (g) (h) CH,CH CHCH CH, CH CH CH,CHCH CH,CHCH CH,CH CH,CH, CH, CH,CH, CH,CH,CH, C(CH,CH),...
-
Give IUPAC names for the following alkenes: (a) (b) (c) (d) (e) (f) OH CI
-
Give IUPAC names for the following compounds. (a) (b) (c) (d) (e) (f) Ph CH3C C CH CH H3C CH3 - (CH3)3C C-CH (CH3)CH2CH3 CH CHC CC-OH CH,CH CH C-C CH
-
Read the article Problem at INSPEECH and answer the question: Why did Inspeech experience a meltdown in 1988?
-
Refer to "Forces Resisting Change". Which of these factors are most strongly affecting Rita and Juan? Support your answer with examples from the scenario.
-
Due to numerous complications involving missed medication dosages, you implement a study to determine the best strategy for enhancing medication adherence. Patients who are on a daily medication...
-
create a data matrix and to code data for analysis by computer. LO9
-
At the beginning of the tax year, Lizzie holds a $10,000 stock basis as the sole shareholder of Spike, Inc., an S corporation. During the year, Spike reports the following. Determine Lizzies stock...
-
17 Question 2 Nichole's furniture store purchased inventory with a 10%, 90 day note payable, for 9,000 on May 1st. On July 30, when the note is due, Nichole will record an interest expense of what...
-
Mrs. Rusholme engaged the firm of Saunders and Watts Ltd. to refinish floors in certain rooms of a home owned by her and her husband in Red Deer, Alberta. She told Mr. Saunders that she and her...
-
Draw and name the seven aldehydes and ketones with the formula C5H10O. Which are chiral?
-
Give structures that fit the following descriptions: (a) An , -un-saturated ketone, C6H8O (b) An -dike tone (c) An aromatic ketone, C9H10O (d) A diene aldehyde, C7H8O
-
Smashers Tennis Club runs coaching courses for its junior members. Each course lasts for ten weeks and is priced at 70 for each junior member. Smashers expects each course to attract 12 junior...
-
Turn this information into an excel sheets with the excel formulas being shown P10.1 (LO 1) (Depreciation for Partial Period-SL, SYD, and DDB) Alladin Company purchased Machine #201 on May 1, 2025....
-
You are the Financial Analyst at Wellington Laboratories Ltd., a New Orleans, USA based bulk drugs manufacturer, which is evaluating the following project for manufacturing a new compound. Year Cash...
-
A variable mesh screen produces a linear and axisymmetric velocity profile as indicated below in the air flow through a 2-ft diameter circular cross section duct. The static pressures upstream and...
-
A vertical round steel rod 2 m long is securely held at its upper end. A weight can slide freely on the rod and its fall is arrested by a stop provided at the lower end of the rod. When the weight...
-
8) Determine the magnitudes of the forces F and P so that the single equivalent couple (i.e. the resultant of the three couples) acting on the triangular block is zero. Z -F F 3 m 10 N, 30 6 m 10 N 3...
-
Selling and Marketing are two Different Things Synopsis Selling and marketing are two different things. The challenge in internal auditing is to communicate key messages to different audiences. Ten...
-
Write a program that initializes an array. It inputs a value from the user and searches the number in the array.
-
Use bond enthalpies from Table 10.3 to determine whether CH 4 (g), CH 3 OH(g), H 2 CO(g), or HCOOH(g) produces the most energy per gram when burned completely in O 2 (g) to give CO 2 (g) and H 2...
-
Compound A has the molecular formula C6H12O3 and shows a strong IR absorption peak at 1710 cm-1. When treated with iodine in aqueous sodium hydroxide, A gives a yellow precipitate. When A is treated...
-
The following is an example of a reaction sequence developed by Derin C. D'Amico and Michael E. Jung (UCLA) that results in enantiospecific formation of two new chirality centers and a carbon-carbon...
-
Additional evidence for the halogenation mechanisms that we just presented comes from the following facts: (a) Optically active 2-methyl-1-phenylbutan-1-one undergoes acid-catalyzed racemization at a...
-
Suppose Universal Forests current stock price is $59.00 and it is likely to pay a $0.57 dividend next year. Since analysts estimate Universal Forest will have a 13.8 percent growth rate, what is its...
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
1. Use the Excel file Asset Allocation Data to determine the following: a.Variances for the individual assets b. Standard deviations for the individual assets c.Covariances between each pair of...

Study smarter with the SolutionInn App


