Give the major product of each of the following reactions: a. b. c. d. e. f. g.
Question:
Give the major product of each of the following reactions:
a.
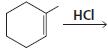
b.
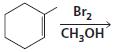
c.
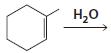
d.
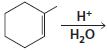
e.
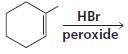
f.
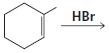
g.
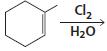
h.
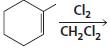
HCI CH3OH H20 Η, H+ H20 HBr peroxide HBr Cl2 H2O Cl2 CH2CI
Step by Step Answer:
a b c No rea...View the full answer



Related Video
Hydrogen peroxide can be used as a mild antiseptic to curb superficial skin infections such as athlete’s foot, but only in diluted quantities. To combat stinky feet, try soaking your feet in a solution of 1 part 3% hydrogen peroxide and 3 parts warm water for 15-20 minutes, then drying them thoroughly. This will kill odor-causing bacteria and soften your feet. To treat athlete\'s foot, you can use a similar solution, but only in diluted quantities, and soak your feet for 30 minutes. Hydrogen peroxide can also be used to keep vegetables fresh by adding 1/4 cup to a bowl of cold water, soaking the vegetables for 20-30 minutes, then draining, drying, and refrigerating them. Alternatively, you can spray vegetables with a solution of 3% hydrogen peroxide and let them stand for a few minutes before rinsing and drying. To keep leftover salad fresh, spray it with a solution of 1/2 cup water and 1 Tbsp. 3% hydrogen peroxide, drain, cover, and refrigerate.
Students also viewed these Organic Chemistry questions
-
Give the major product of each of the following reactions: a. b. c. d. e. f. HBr HC CCH3 peroxide excess Br 3 CH2Cl2 excesS CHC CCH HBr excess
-
Give the major product of each of the following reactions a. b. c. d. e. f. g. h. i. CCH3 + HNO3 S NO2 CHs CH CHCH NCH CH 3 CH3 HO CH3NCH3 + PC15- NC 1. H202 2. CH3 1. HO 3. Ht CH3 + CH:CH2MgBr 2.4...
-
Give the expected major product of each of the following reactions. PCC is the abbreviation for pyridinium chlorochromate (Section 8-6). (a) (b) (c) (d) (e) CH CH CH,OH NeCrO HSO, HO PCC, CH,CI (CH3)...
-
Formulate a plausible mechanism for the following reaction. The product is a precursor of mediquox (shown in the margin), an agent used to treat respiratory infections in chickens (no, we are not...
-
Highcountry.com is an Internet retailer of sporting good products. Customers order sporting goods from the company, using an online catalog. The company processes these orders and delivers the...
-
Describe the distribution requirements of the IRC with respect to retirement accumulations.
-
Do you think a cost focus or a differentiation focus strategy might be more appropriate? Explain.LO1
-
Muskoge Company uses a process-costing system. The company manufactures a product that is processed in two departments: Molding and Assembly. In the Molding Department, direct materials are added at...
-
X came India for first time on July 24, 2014. From July 24, 2014 to December 25, 2015 he was in India. Again, he came to India on August 5, 2018 for employment purpose & left India on November 25,...
-
1. What did Mary's autocorrelation analysis show? 2. Fit an appropriate smoothing procedure to Mary's data, examine the residual autocorrelations, and generate forecasts for the remainder of...
-
For each pair of bonds, which has the greater strength? Briefly explain why. a. b. c. d. CH,-Cl or CH,-Br CH,CH2CH2 or CH,CHCH3 CH, CH or CH, CH,CH, I-Br or BrBr
-
Using any alkene and any other reagents, how would you prepare the following compounds? a. b. c. d. e. f. CH3CH CH2CHCH CI CH2CHCH3 CH3CH2CHCHCH2CH3 Br CH CH2CHCHCH2CH3 Br Cl
-
What is Long Form Audit Report?
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
Some telephoto cameras use a mirror rather than a lens. What radius of curvature mirror is needed to replace a 800 mm focal length telephoto lens?
-
Catherine (aged 42) and Johnson (aged 45) have been married for 12 years. Johnson is a project manager of an event company at a monthly salary of $55,000 with an additional one-month salary of...
-
Trans-1-Phenyl-1, 3-butadiene has max = 280 ( = 27,000) calculate the concentration of a solution that has A = 0.643 at 280nm in a 1 cm cell.
-
Nitro methane max = 275nm ( = 1.5) what kind of transition is responsible for this absorption?
-
3-Buten-2-one has max =213nm ( = 7080) and max = 320nm ( = 21) what kind of transition is responsible for each of these absorptions?
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


