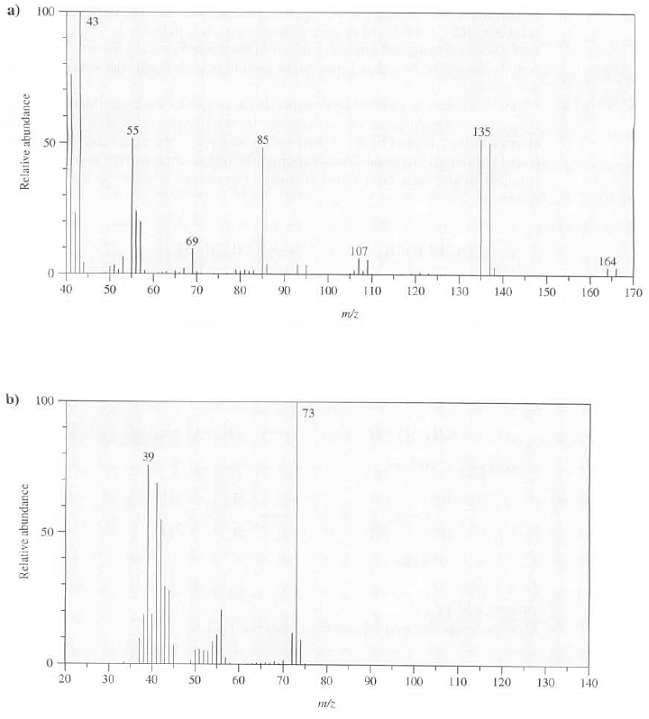
What conclusions can be drawn about these compounds from their massspectra? 100 43 55 135 85 69
Question:
What conclusions can be drawn about these compounds from their massspectra?
Transcribed Image Text:
100 43 55 135 85 69 107 164 trpt 40 50 60 70 80 L10 120 130 90 100 140 150 160 170 m/z b) 100 73 50 20 30 40 50 60 70 80 90 100 110 120 130 140 Relative abundance Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
a This similar intensities of the M mz 164 and th...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What conclusions can be drawn about income and health inequality between countries in the world? What is the general relationship between income and health status? Why do you think there is such a...
-
What conclusions can be drawn from a feasibility analysis?
-
What conclusions can be drawn from a study with a null result?
-
96. A 66-year-old woman with a long history of heavy smoking presents to her doctor with complaints of shortness of breath and chronic coughing that has been present for about 2 years and has been...
-
While performing interim audit procedures of accounts receivable, numerous unexpected errors are found resulting in a change of risk assessment. Which of the following audit responses would be most...
-
In a network using the Selective-Repeat protocol with m = 4 and the sending window of size 8, the value of variables are S f = 62, S n = 67, and R n = 64. Packet 65 has already been acknowledged at...
-
5 Cmo clasificara a Walmart en referencia a su posicin en la rueda de detallistas en comparacin con un detallista off-price ?
-
Calculate the discount rate consistent with a cap rate of 12 percent and a growth rate of 6 percent. Show how your answer would change if the cap rate dropped to 10 percent while the growth rate...
-
19. Dr. Smith purchased a bond on April 1, 2019, at par of $100,000 plus accrued interest of $1,500, for a total purchase price of $101,500. On December 31, 2019, Dr. Smith collected the $6,000...
-
A $100,000 investment with a zero scrap value has an 8-year life. Compute the payback period if straight-line depreciation is used and net income is determined to be $20,000.
-
Predict the relative intensities of the M +, M + 2, and M + 4 peaks for these compounds. Assume that 79Br/81Br = 1/1 and 35Cl/37Cl=3/1. (a) CH2Cl2 (b) CH2BrCl
-
Show the molecular ions formed from these compounds: b) a) CH,NHCH,
-
Why would a company purchase a subsidiary rather than simply establish a new subsidiary ofi ts own?
-
Match each of the following transactions of Lesch \& Company with the appropriate letters, indicating the debits and credits to be made. The key for the letters follows the list of transactions. The...
-
Workers act as sellers of their time in the labor market in return for some wage. Lets discover your individual supply curve for labor. For each hourly wage rate provided in the accompanying table,...
-
The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) monitors and evaluates health care providers according to strict standards and guidelines. Improvement in the quality of care...
-
The U.S. National Highway Traffic Safety Administratio (NHTSA) independently tests over 2,400 types of tires annually. In 2015, they issued more than 900 recalls, affecting 51 million vehicles...
-
The Hudson Jewelers case study can be found in Appendix C. Chapter 17 Case Questions for Discussion: 1.Research and acquire the criteria for diamond appraisals and critique these criteria in terms of...
-
What percent of females thought that they were almost certain to be married in the next ten years? (a) About 16% (c) About 40% (e) About 61% (b) About 24% (d) About 45% 30. Your percent from the...
-
What are current assets and current liabilities? How are they different from non-current assets and non-current liabilities?
-
In 1998, Robert Rubin, then the U.S. Treasury Secretary, claimed that the billions of dollars the U.S. government regularly provides to the IMF to make loans to other nations cost U.S. taxpayers not...
-
Write (+ and (- by the appropriate atoms and draw a dipole moment vector for any of the following molecules that are polar: (a) HF (b) IBr (c) Br2 (d) F2
-
Write bond-line formulas for (a) Four aldehydes and (b) Three ketones that have the formula C5H10O.
-
Write bond-line formulas for four carboxylic acids with the formula C5H10O2.
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


