Show the molecular ions formed from these compounds: b) a) CH,NHCH,
Question:
Show the molecular ions formed from these compounds:
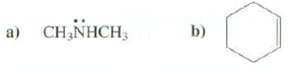
Transcribed Image Text:
b) a) CH,NHCH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (19 reviews)
a C...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Compounds A and B are isomeric amines of molecular formula C8H11N. Identify each isomer on the basis of the 1H NMR spectra given in Figure 22.9. Question continue over next page Figure 22.9 Compound...
-
Compounds A and B are isomers of molecular formula C9H19Br. Both yield the same alkene C as the exclusive product of elimination on being treated with potassium tert-butoxide in dimethyl sulfoxide....
-
Compounds A and B are isomers of molecular formula C9H19Br. Both yield the same alkene C as the exclusive product of elimination on being treated with potassium tert-butoxide in dimethyl sulfoxide....
-
?
-
Identify the major stakeholders in your organization, discuss there different types.
-
Suppose that the total cost (in dollars) for a product is given by C(x) = 1500 + 200 ln (2x + 1) where x is the number of units produced. (a) Find the marginal cost function. (b) Find the marginal...
-
In order to compare the means of two populations, independent random samples of 400 observations are selected from each population, with the following results: Sample 1 Sample 2 a. Use a 95%...
-
Ramrod, Inc., sells a warehouse for $350,000. It purchased the warehouse 10 years ago for $250,000 and had taken $75,000 in depreciation on the building to the date of sale. Identify the tax issue(s)...
-
Green Thumb Gardening is a small gardening service that uses activity - based costing to estimate costs for pricing and other purposes. The proprietor of the company believes that costs are driven...
-
There are only two stations, A and B, in a bus 1-persistence CSMA/CD network with T p = 25.6 μs and T fr = 51.2 μs. Station A has a frame to send to station B. The frame...
-
What conclusions can be drawn about these compounds from their massspectra? 100 43 55 135 85 69 107 164 trpt 40 50 60 70 80 L10 120 130 90 100 140 150 160 170 m/z b) 100 73 50 20 30 40 50 60 70 80 90...
-
Show equations for the major fragmentations you would expect from the molecular ions of these compounds. List the m/z of the productions. CH3 CH2 a) CH;CH-CH,CH-CH; b) CH;CH,CHCH2CH3
-
Find the critical numbers of the function. g() = 4 - tan
-
how could a government or world leader have used ERM to respond to one of the financial, operational, or governance aspects of the covid19 pandemic? include references for further reading.
-
Computing and Interpreting Return on investment Selected operating data for two divisions of Outlook Brewing, Ltd., of Australia are given below: Division Queensland New South Wales Sales: $4,000,000...
-
Consider a parcel of land that contains an even ages stand of trees currently of age in A in t=0. you have to decide how much longer to allow this stand to grow given that when you cut the stand, you...
-
What does the company report for the following accounts for the most current fiscal year:Enter your answer in thousands.a . Cash$fill in the blank 1 1 , 1 5 4 , 8 6 7 b . Short - term investments (...
-
Consider the translational mechanical system with a nonlinear spring shown below. The spring is defined by s(t)=ks(t), where x(t) is the spring length and f(t) the spring force. Nonlinear spring 0000...
-
Discuss whether the large amounts of money invested in high-profile sporting events would better serve the community in other ways.
-
Selected condensed data taken from a recent statement of financial position of Morino Ltd. are as follows. MORINO LTD. Statement of Financial Position (partial) Other current assets...
-
The World Bank was established in 1944. To date, it has lent nearly $390 billion, mostly to poor nations. However, its accomplishments have been questioned. For example, since 1951, India has...
-
Write bond-line formulas for three esters with the formula C5H10O2.
-
Write another resonance structure for ethyl acetate. Include formal charges.
-
Write another resonance structure for acetamide.
-
The plant asset and accumulated depreciation accounts of Pell Corporation had the following balances at December 3 1 , 2 0 2 0 : Transactions during 2 0 2 1 were as follows: a . On January 2 , 2 0 2...
-
All else equal, a company's P/E ratio will ____________ when the discount rate ___________. rise, rise fall, rise fall, falls cannot be determined All else equal, a company's P/E ratio will _____...
-
The cost of partially completed goods at the end of the period would be Ending work in process inventory Cost of goods sold Beginning finished goods inventory Beginning work in process inventory

Study smarter with the SolutionInn App


