Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is
Question:
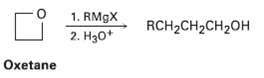
Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene oxide. Suggest a reason for the difference in reactivity between oxetane and ethyleneoxide.
Transcribed Image Text:
1. RM9X 2. H30* RCH2CH2CH2OH Oxetane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
The mechanism of Grignard addition to oxetane is the same as the mec...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The rate of debranching is much slower than that of phosphorolysis. Explain how highly branched glycogen molecules release glucose-1-phosphate at a greater rate than relatively unbranched ones.
-
Grignard reagents react slowly with oxetane to produce primary alcohols. Propose a mechanism for this reaction, and suggest why oxetane reacts with Grignard reagents even though most ethers do not....
-
The reaction of Grignard reagents with nitriles is another method of preparing ketones. The example of this synthesis is shown in Fig. P21.57. Identify compound A, and give a mechanism for its...
-
Suppose you want to reduce the level of trash disposed by your household. Develop an emission standard, a technology standard, and an ambient standard that would accomplish the reduction.
-
In today's global business environment, does the physical location of a business matter?
-
In each of the following situations, what is the amount of profit or loss? In each situation, what account will be debited and credited, and for what amount, in the journal entry to close the Income...
-
2. Penang Corporation acquired a 90 percent interest in Doki Corporation on January 1, 2012, when the book value of Dokis net assets was equal to their fair value. During 2014, Doki sold land that...
-
Suppose the following data are derived from the 2014 financial statements of Southwest Airlines. (All dollars are in millions.) Southwest has a December 31 year-end. Cash balance, January 1,...
-
A firm expects to sell 25,400 units of its product at $11.40 per unit and to incur variable costs per unit of $6.40. Total fixed costs are $74,000. The total contribution margin is Muriple choice...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Acid-catalyzed hydrolysis of a 1, 2-epoxycyclohexane produces a trans-diaxial 1, 2diol. What product would you expect to obtain from acidic hydrolysis of cis-3-tert-butyl-1, 2-epoxycyclohexane?...
-
Treatment of trans-2-chlorocyclohcxanol with NaOH yields 1, 2-epoxycyclo- hexane, but reaction of the cis isomer under the same conditions yields Cyclohexanone. Propose mechanisms for both reactions,...
-
A charge of 100 lbmol of 35 mol% n-hexane, 35 mol% nheptane, and 30 mol% n-octane is to be distilled at 1 atm in a batch rectifier, consisting of a still-pot, a column, and a total condenser, at a...
-
226 Payroll Accounting Chapter 7: Comprehensive Projects-Paper-Based Versions One-Month Project NOTE! Templates needed to complete these exercises, including one containing year-to-date payroll data,...
-
The Westchester Chamber of Commerce periodically sponsors public service seminars and programs Currently, promotional plans ore under way for this year's program Advertising alternatives include...
-
Mastery Problem: Activity-Based Costing WoolCorp WoolCorp buys sheep's wool from farmers. The company began operations in January of this year, and is making decisions on product offerings, pricing,...
-
The following system of linear equations is called underdetermined because there are more variables than equations. x2x 3x3 = 4 2x1x2 + 4x3 = -3 Similarly, the following system is overdetermined...
-
Write a 2000-word Reflection paper on " Country Managers Simulation by considering the following points: Countries chosen during the simulation were Argentina and Brazil: 1. Explain why you did what...
-
To distinguish the rights of both employers and employees under the At - Will Employment doctrine.
-
Classify each of the following activities as proper or prohibited under the various consumer statutes you have studied. a. Calling a hospital room to talk to a debtor who is a patient there. b....
-
One mole of carbon dioxide is to be compressed adiabatically from 1 bar and 25 C to 10 bar. Because of irreversibilities and poor design of the compressor, the compressor work required is found to...
-
Write a Lewis structure for each of the following: (a) HF (b) F2 (c) CH3F (d) HNO2 (e) H2SO3 (f) BH4 (g) H3PO4 (h) H2CO3
-
Write bond-line structural formulas for (a) Two constitutionally isomeric primary alkyl bromides with the formula C4H9Br, (b) A secondary alkyl bromide, and (c) A tertiary alkyl bromide with the same...
-
Although we shall discuss the naming of organic compounds later when we discuss the individual families in detail, one method of naming alkyl halides is so straightforward that it is worth describing...
-
All else constant, if the yield to maturity of a bond increases, the the value of the bond __________. a. increases b. decreases c. remains the same d. not enough information To answer enter a, b, c,...
-
Martha s Vineyard Marine Supply is a wholesaler for a large variety of boating and fishing equipment. The company s controller, Mathew Knight, has recently completed a cost study of the firm s...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not

Study smarter with the SolutionInn App


