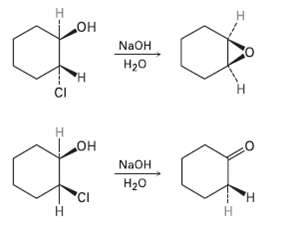
Treatment of trans-2-chlorocyclohcxanol with NaOH yields 1, 2-epoxycyclo- hexane, but reaction of the cis isomer under the
Question:
Treatment of trans-2-chlorocyclohcxanol with NaOH yields 1, 2-epoxycyclo- hexane, but reaction of the cis isomer under the same conditions yields Cyclohexanone. Propose mechanisms for both reactions, and explain why the different results areobtained.
Transcribed Image Text:
н но° N2OH H20 н но NAOH Н2о 'CI Н Н Н т
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
H OH trans2Chloro cyclohexanol ring flip CI H OH cr H H 12Epoxyeyelohexane H cis2Chl...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose mechanisms for the reactions shown in Problems 22-62 parts (a) and (b) and 22-63 parts (a) and (b). In problem 22-62 (a) (b) In problem 22-63 (a) (b) CH3 TOH CH CH2-C-H CH CH OCH3 CH3OH CH3...
-
Propose a mechanism for the acid-catalyzed reaction of cyclohexanone with pyrrolidine.
-
Propose reasonable fragmentation mechanisms that explain why The EI mass spectrum of benzoic acid shows major peaks at m/z = 105 and m/z = 77.
-
The costs of achieving emission reductions in the future will depend greatly on the types of policies used to reduce emissions today. Explain.
-
Which of Porter's Five Forces did Apple address through the introduction of the iPhone and customer developed iPhone applications?
-
On December 31, 2019, the board of directors of Indiana Corporation voted to appropriate $150,000 of retained earnings each year for five years to establish a reserve for contingencies. Give the...
-
3. Ping Corporation sold equipment with a five-year remaining useful life to its 80 percent-owned subsidiary, Song Corporation, on January 1, 2014. The equipments cost was $300,000, and it was sold...
-
In 2015, Ginger Graham, age 46 and wife of Greg Graham engaged in the transactions described below. Determine Gingers gift tax liability for 2015 if she and Greg elect gift splitting and Greg gave...
-
Management of Mittel Rhein AG of Kln, Germany, would like to reduce the amount of time between when a customer places an order and when the order is shipped. For the first quarter of operations...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene oxide. Suggest a reason...
-
Ethers undergo an acid-catalyzed cleavage reaction when treated with the Lewis acid BBr3 at room temperature. Propose a mechanism for thereaction. + CHBr CH 1. r 2. H20
-
1. Is there federal jurisdiction? 2. What states have jurisdiction? (Assume subject matter jurisdiction requires the incident or the residents of all defendants to be in the state of suit.)
-
Why would a company pursuing vertical integration need to develop a multibusiness model? to determine the scope and boundaries of the new firm to explain how to successfully compete within a single bu
-
In the following vignette write out three examples of a MI intervention in response to what the client has shared. Mariana is a 31-year-old, married Hispanic female who works as an advertising...
-
A small factory has two types of loads (balanced 3 phase Wye connected). Lighting accessories with a total power of 6 kW at unity power factor. Two electric machines rated 13.5 kVA each operating at...
-
Which terms are used to determine severity of mental illness /Disorder in a DSM diagnosis? I Superficial, Typical, Intense Mild, Moderate, Severe Low, Medium, High Level I, Level II, Level II
-
Target Inventory You are the operations manager of a firm that uses the continuous inventory control system. Suppose the firm operates 50 weeks a year, 350 days, and has the following characteristics
-
Do you think that the requirement that servers be attractive to males is a bona fide occupational requirement, and necessary for the continued successful operation of The Cruz Cantina?
-
Before the 1973 oil embargo and subsequent increases in the price of crude oil, gasoline usage in the United States had grown at a seasonally adjusted rate of 0.57 percent per month, with a standard...
-
Hydrogen has an auto-ignition temperature of 853 K; that is, hydrogen will ignite spontaneously at that temperature if exposed to oxygen. Hydrogen is to be adiabatically and reversibly compressed...
-
Write bond-line structural formulas for (a) Two primary alcohols, (b) A secondary alcohol, and (c) A tertiary alcohol-all having the molecular formula C4H10O.
-
One way of naming alcohols is to name the alkyl group that is attached to the -OH and add the word alcohol. Write bond-line formulas for (a) Propyl alcohol and (b) Isopropyl alcohol.
-
One way of naming ethers is to name the two alkyl groups attached to the oxygen atom in alphabetical order and add the word ether. If the two alkyl groups are the same, we use the prefix di-, for...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...

Study smarter with the SolutionInn App


