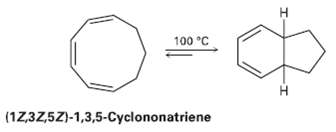
Heating (1Z, 3Z, 5Z)-1, 3, 5-cyclorionatriene to 100?C causes Cyclization and formation of a bicyclic product. Is
Question:
Heating (1Z, 3Z, 5Z)-1, 3, 5-cyclorionatriene to 100?C causes Cyclization and formation of a bicyclic product. Is (he reaction conrotatory or disrotatory? What is the stereo chemical relationship of the two hydrogen?s at the ring junctions, cis or trans?
Transcribed Image Text:
H. 100 °C (17,3Z,5Z)-1,3,5-Cyclononatriene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
The cyclononatriene is a 6a electro...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What is the stereo chemical relationship of D-ribose to L-xylose? What generalizations can you make about the following properties of the two sugars? (a) Melting point (b) Solubility in water (c)...
-
What are the stereo chemical configurations of the two diastereomers of (25, 4R) -2, 4-octanediol?
-
What is the stereo chemical configuration of the enantiomer of (2S, 4R)-2, 4-oclanediol?
-
Explain why each of the following statements about profit-maximizing competitive firms is incorrect. Restate each one correctly. a. A competitive firm will produce output up to the point where price...
-
From publicly available information, find at least two examples of activist investors influencing the policies or strategies of a firm? Describe the impact you believe they had in each instance?
-
What are STRs? Why are they sometimes called microsatellites?
-
Why dont we rebalance the test data set?
-
Consider Managerial Practice and the discussion of Steinways approach to achieving top quality. To get a better idea of the craft oriented production process. Visit www.stelnwacom/factorytours.html....
-
Part III. [25 pts] Bivariate Regression Following on the previous example, and using the descriptive statistics on this student level dataset, and regression output below, please answer the following...
-
A wealthy couple have three children named Xena, Yuri, and Zoe. To give their children a lesson in entrepreneurship, the parents have decided to invest a total of $35,000. They asked their children...
-
Would you expect the following reaction to proceed in a conrotatory or disrotatory manner? Show the stereochemistry of the Cyclobutene product, and explain youranswer. hv
-
(2E, 4Z, 6Z, 8E)-2, 4, 6, 8-Decatetraene has been cyclized to give 7, 8-dimethyl-1, 3, 5-cyclooctatriene. Predict the manner of ring closureconrotatory or disrotatoryfor both thermal and...
-
In a test of the effectiveness of a new battery design, 16 battery-powered music boxes are randomly provided with either the old design or the new version. Hours of playing time before battery...
-
1. How will you check if a class is a child of another class? 2. What is init method in python?
-
1. What are lists and tuples? What is the key difference between the two? 2. What is Scope in Python?
-
1. What is an Interpreted language? 2. What is a dynamically typed language?
-
Q.1 If denotes increasing order of intensity, then the meaning of the words [talk shout scream] is analogous to [please pander]. Which one of the given options is appropriate to fill the blank? (A)...
-
Discuss the qualities of voice so that all participants are clear on the meaning of rate, pitch, and volume.
-
B.) What is the approximate concentration of free Zn 2+ ion at equilibrium when 1.0010 -2 mol zinc nitrate is added to 1.00 L of a solution that is 1.080 M in OH - . For [Zn(OH) 4 ] 2- , K f = 4.610...
-
The SIP evolved from the ticker tapes providing price and volume data from the exchanges. It is said that now, the SIP is far busier than it was more than a century ago when the ticker tapes were...
-
The compound KSCN is a source of thiocyanate ion. (a) Write the two most stable Lewis structures for thiocyanate ion and identify the atom in each that bears a formal charge of -1. (b) Two...
-
Reaction of ethyl iodide with triethylamine [CH3H2)3N:] yields a crystalline compound C8H20N in high yield. This compound is soluble in polar solvents such as water but insoluble in non polar ones...
-
Write an equation, clearly showing the stereochemistry of the starting material and the product, for the reaction of (S)-1-bromo-2-methylbutane with sodium iodide in acetone. What is the...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


