How many different absorption bands would appear in the 13C-NMR spectra of thesecompounds? b) CH;CH,CH,CH,CH3 c) CH
Question:
How many different absorption bands would appear in the 13C-NMR spectra of thesecompounds?
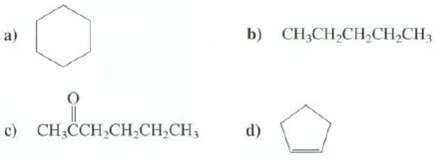
Transcribed Image Text:
b) CH;CH,CH,CH,CH3 c) CH CCH,CH;CH,CH, d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
The number of absorptions in the 13CNMR spec...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The proton-decoupled 13C NMR spectra of 3-heptanol (A) and 4-heptanol (B) are given in Fig. 13.22 on page 626. Indicate which compound goes with each spectrum, and explain your reasoning. Fig. 13.22...
-
How many signals would you expect in the 13 C NMR spectrum of each of the compounds in Problem 16.34? In Problem 16.34 How many signals would you expect in the 1 H NMR spectrum of each of the...
-
The 1H and 13C NMR spectra of compound A, C8H9Br are shown. Propose a structure for A, and assign peaks in the spectra to your structure. TMS O ppm 10 8. 6. Chemical shift (8) TMS 200 180 160 140 120...
-
Is this a confined or unconfined aquifer? Please explain. 100 m amsl 78 m amsl 56 m amsl 48 m amsl A 50 m 11m B Clay Sand Clay 100 m
-
On January 1, 2011, Rand Corp. issued shares of its common stock to acquire all of the outstanding common stock of Spaulding Inc. Spaulding's book value was only $140,000 at the time, but Rand issued...
-
Construct a frequency histogram for the data. Consider the data in the frequency table, and use these data. Score Frequency 1.............................4 2.............................6...
-
Find the appropriate values of n, and n2 (assume n, = n2) needed to estimate (p, - p,) with: a. A bound on the error of estimation equal to 3.2 with 95% confidence. From prior experience it is known...
-
Martell Mining Companys ore reserves are being depleted, so its sales are falling. Also, its pit is getting deeper each year, so its costs are rising. As a result, the companys earnings and dividends...
-
No adjustments are necessary for accounting numbers when making forecasts of a Profit and Loss Statement, Balance Sheet, Cash Flow Statement as they are always correct and prepared to highest ethical...
-
The Professional Golf WH Association (PGA) and Golf Digest have developed the Play Golf America program, in which teaching professionals at participating golf clubs provide a free 10-minute lesson to...
-
Predict the 1H.NMR spectra of these compounds include the approximate chemical shift, multiplicity, and integral for each type ofhydrogen. CI b) CH;CHCH; ) C,CH,H c) CH,CH,OCH,CH3 CH2CH2NO2 f)...
-
Assign the absorptions in the 13C-NMR spectra of these compounds to the appropriate carbons: (a) 1-Butanol; absorptions at 61.4, 35.0, 19.1, and 13.6 (b) Cyclohexanone; absorptions at 209.7, 41.9,...
-
The number of telecommuters in the United States did not grow as fast during the 1990s as some had predicted it would. Provide a rationale for why you think telecommuting has increased more rapidly...
-
Woodland Wearables produces two models of smartwatches, the Basic and the Flash. The watches have the following characteristics:Basic Flash Selling price per watch$ is 270$ 460 Variable cost per...
-
Based on the information provided and recognizing the value of coordinating across its portfolio of businesses, how should LendingTree manage these newer businesses? * as more integrated units * as...
-
Trust Fund Worksheet Background An inter vivos trust was created by Isaac Posney. Isaac owned a large department store in Juggins, Utah. Adjacent to the store, Isaac also owned a tract of land that...
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Gracia Enterprises operates across five industries. Task 1 : After reviewing the information provided, determine which of the five operating segments are reportable based on the revenue test, asset...
-
understand the legal principles governing the commercial regulation of sport such as intellectual property rights and match fixing.
-
The Zwatch Company manufactures trendy, high-quality moderately priced watches. As Zwatch's senior financial analyst, you are asked to recommend a method of inventory costing. The CFO will use your...
-
At FedEx, the marginal cost incurred in delivering packages speedily often encompasses more than just the expense of physically transporting a package from one location to another. The additional...
-
Have the following reactions taken place in a conrotatory or disrotatory manner? Under what conditions, thermal or photochemical, would you carry out eachreaction? (a) (b) --
-
What stereochemistryantarafacial or suprafacialwould you expect to observe in the following reactions? (a) A photo chemical [1, 5] sigma tropic rearrangement (b) A thermal [4 + 6] cyclo addition (c)...
-
The following thermal isomerization occurs tinder relatively mild conditions. Identify the pericyclic reactions involved, and show how the rearrangementoccurs. CH C- CeH5. C&H5 "CH CD CoH5 CgH5 CEH5...
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll Note: Round your answers to the nearest hundredth
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Golden Corporation's current year income statement, comparative balance sheets, and...
-
Glencove Company makes one model of radar gun used by law enforcement officers. All direct materials are added at the beginning of the manufacturing process. Information for the month of September...

Study smarter with the SolutionInn App


