How might the following amines be prepared using reductive amination reactions? Show all precursors if more than
Question:
How might the following amines be prepared using reductive amination reactions? Show all precursors if more than one ispossible.
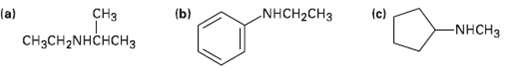
Transcribed Image Text:
CHз (b) NHCH2CH3 (c) (a) CHзCH2NHCHсHз -NHCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
Look at the target molecule to find the groups bonded to nitrogen One group ...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How might the following compounds be prepared using Michael reactions? Show (he nucleophilic donor and the electrophilic acceptor in eachcase. ", , (b) . (a) CHCCH-CH2CH2CH3 CH2H2CCgHs o2Et NO2 (d)...
-
How might the following business specialists use learning curves: accountants, marketers, financial analysts, personnel managers, and computer programmers?
-
How might the following influences affect a firms business risk (consider each separately)? a. Imports increase the level of competition b. Labor costs decline c. Health care costs (provided for all...
-
DRAW A PLAN OF YOUR HOME THEN ESTIMATE QUANTITY OF * FOOTING *BLOCK WALL UNDER DPC * BLOCK WALL ABOVE DPC * CEMENT PLASTERING OUTSIDE *GYPSUM PLASTERING INSIDE
-
How can organizations motivate employees to promote safety and health in the workplace?
-
Given the data in the file Stock Beta.xlsx, estimate the beta (and alpha) for Microsoft (MSFT). Do this for each criterion and each period of time to obtain a table analogous to that in the top right...
-
Does it matter whether the surcharge is called a gratuity or a service charge? How would that be determined?
-
Refer to the information in QS. Assign costs to the assembly departments outputspecifically, the units transferred out to the painting department and the units that remain in process in the assembly...
-
Crestline, Inc. is a manufacturing firm that allocates overhead on the basis of direct labor hours (DLH) at a rate of $16 per DLH. The job cost record for Job 23 for 20 units shows the following:...
-
1. Compare and contrast the various segments of Chinese luxury consumers and customers profiled in the case. 2. How have luxury goods brands responded to President Xi Jinpings crackdown on...
-
Show two methods for the synthesis of dopamine, a neurotransmitter involved in regulation of the central nervous system. Use any alkyl halideneeded. Dopamine
-
How could you prepare the following amine using a reductive aminationreaction?
-
Where is the premium or discount on bonds payable presented on the balance sheet?
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a converging lens, closer to the lens than to the focal point. (b) Is the image...
-
Power efficiency has become very important for modern processors, particularly for embedded systems. Create a version of gcc for two architectures that you have access to, such as x86, RISC-V,...
-
There is a movement toward wireless mobile computing using thin-client technology. Go to the Web and visit some of the ma jor computer vendors that are producing thin-client products such as handheld...
-
Draw a B-tree of order 4 and height 3 containing the fewest elements. Show an example of a split that would be applied by inserting the fewest number of elements.
-
Repeat Example 10-4, except calculate the diameter at the bottom of the column. Example 10-4 A distillation column is separating n-hexane from n-heptane using 1-in. ceramic Intalox saddles. The...
-
Nontraditional hospitality industry workers include the following: LO1 A. Displaced homemakers B. Recent immigrants C. Retirees D. All of the above
-
An auto-parts manufacturer is considering establishing an engineering computing center. This center will be equipped with three engineering workstations each of which would cost $25,000 and have a...
-
An equilibrium mixture contains N 2 O 4 (P = 0.28 atm) and NO 2 (P = 1.1 atm) at 350 K. The volume of the container is doubled at constant temperature. Write a balanced chemical equation for the...
-
Suggest which of the following monomers might polymerize well on treatment with BF3. (a) Vinyl chloride (b) Vinyl acetate (c) Methyl a-cyanoacrylate
-
Chain branching occurs in cationic polymerization much as it does in free-radical polymerization. Propose a mechanism to show how branching occurs in the cationic polymerization of styrene. Suggest...
-
Draw the important resonance forms of the stabilized anion formed in the anionic polymerization of methyl acrylate.
-
On February 1, 2021, Arrow Construction Company entered into a three-year construction contract to build a bridge for a price of $8,600,000. During 2021, costs of $2,200,000 were incurred with...
-
Salespersons' Report and Analysis Walthman Industries Inc. employs seven salespersons to sell and distribute its product throughout the state. Data taken from reports received from the salespersons...
-
Stockholders do not have the power to bind the corporation to contracts. This is referred to as lack of mutual agency. True false question. True False

Study smarter with the SolutionInn App


