How would you prepare the following amines, using both Hofmann and Curtius rearrangements on a carboxylic acidderivative?
Question:
How would you prepare the following amines, using both Hofmann and Curtius rearrangements on a carboxylic acidderivative?
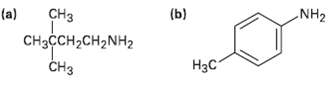
Transcribed Image Text:
CHз (b) (a) NH2 CняҫсH2CH2NH2 CHз Нас
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (17 reviews)
In both of these reactions the product amine is for...View the full answer

Answered By

Stephen ouma
I have worked with different academic writing companies such as wriredom, writerbay, and Upwork. While working with these companies, I have helped thousands of students achieve their academic dreams. This is what I also intend to do here in SolutionInn
4.90+
19+ Reviews
63+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you prepare the following carboxylic acids? (a) (CH 3 ) 3 CCO 2 H from (CH 3 ) 3 CCl (b) CH 3 CH 2 CH 2 CO2H from CH 3 CH 2 CH 2 Br
-
Using any alkyne needed, how would you prepare the following alkenes? (a) Trans-2-Octenc (b) Cis-3-Heptcne (c) 3-Methyl-1-pentene
-
How would you prepare the following diols? (b) (a)
-
The receiver most commonly used in AM and FM radio broadcast is the so-called superheterodyne receiver. Answer the following questions about this receiver. a. Draw the block diagram of a...
-
To identify instances of sexual harassment, the courts may use a "reasonable woman" standard of what constitutes offensive behavior. This standard is based on the idea that women and men have...
-
Change the exchange rate model in Example 7.2 slightly so that the company is now a UK manufacturing company producing for a U.S. market. Assume that the unit cost is now 75, the demand function has...
-
Whom should the restaurant manager and company look to for guidance on property serving temperatures and techniques? Could you defend this source in court?
-
Audit Risk. Your firm recently signed a letter of engagement to audit CitCo, the local city and county government. Over your morning cup of coffee, you open the local newspaper and read the...
-
In process costing system, the predetermined overhead rate is computed O a. separately for each processing department O b. only for the last department where units are completed and transferred to...
-
Jamonit Ltd is a non-group employer which paid wages of $136,000 in the Northern Territory during March 2021. The company does not pay wages in any other state. Calculate the payroll tax payable in...
-
How could you prepare the following amine using a reductive aminationreaction?
-
What products would you expect from Hofmann elimination of the following amines? If more than one product is formed, indicate which ismajor. NH2 (b) NH2 (a) CH3CH2CH2CHCH2CH2CH2CH3 NHCH2CH3 (d) NH2...
-
For the following exercises, identify the conic with a focus at the origin, and then give the directrix and eccentricity. r= 3 44 sin 0
-
As of June 30, 2012, the bank statement showed an ending balance of \(\$ 13,879.85\). The unadjusted Cash account balance was \(\$ 13,483.75\). The following information is available: 1. Deposit in...
-
An engineering study has developed the following cost data for the production of product A: (1) If the current production level is 1,500 units, what is the incremental cost of producing an additional...
-
On December 1, a group of individuals formed a corporation to establish the Local, a neighborhood weekly newspaper featuring want ads of individuals and advertising of local firms. The free paper...
-
Design an arithmetic circuit with one selection variable S and two n-bit data inputs A and B. The circuit generates the following four arithmetic operations in conjunction with the input carry C in ....
-
For the system you chose for Problems and Exercises 3, complete section 4.0, A-C, Management Issues, of the BPP Report. Why might people sometimes feel that these additional steps in the project plan...
-
In order to provide quality products and services to customers and guests, employees must: LO1 A. Be paid higher than average industry wages B. Have formal hospitality industry education C. Be...
-
Using (1) or (2), find L(f) if f(t) if equals: t cos 4t
-
A sample of CaCO 3 (s) is introduced into a sealed container of volume 0.654 L and heated to 1000 K until equilibrium is reached. The K p for the reaction CaCO 3 (s) CaO(s)+ CO 2 ( g) is 3.9 * 10 -2...
-
The ammonium ion, NH4+, has a tetrahedral geometry analogous to that of methane. Explain this structure in terms of atomic and molecular orbitals.
-
Use lines, dashed wedges, and solid wedges to show the geometry of CF4 and CH3SH.
-
Silicon is just below carbon in the periodic table. Predict the geometry of silicon tetrafluoride, SiF4.
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


