How would you prepare the following carbonyl compounds starting from an alkyne (reddish brown ? Br)? (b)
Question:
How would you prepare the following carbonyl compounds starting from an alkyne (reddish brown ? Br)?
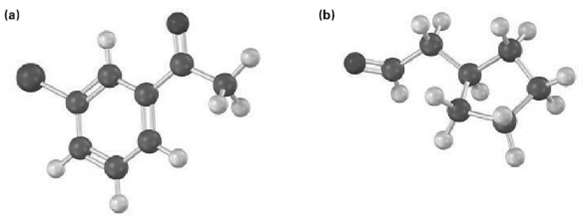
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (20 reviews)
a Br b HC ...View the full answer

Answered By

Asd fgh
sadasmdna,smdna,smdna,msdn,masdn,masnd,masnd,m asd.as,dmas,dma.,sd as.dmas.,dma.,s ma.,sdm.,as mda.,smd.,asmd.,asmd.,asmd.,asm
5.00+
1+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you prepare the following carbonyl compounds from anitrile? ( (a) CH3CH2CCH2CH3 CH O2N
-
How would you prepare the following compounds from the given starting materials? a. b. c. d. CH3CH2CH CH3CHCH N(CH3)2 CH CHCH OCH3
-
Using any alkyne needed, how would you prepare the following alkenes? (a) Trans-2-Octenc (b) Cis-3-Heptcne (c) 3-Methyl-1-pentene
-
Assume that Polaris manufactures and sells 60,000 units of a product at $ 11,000 per unit in domestic markets. It costs $ 6,000 per unit to manufacture ($ 4,000 variable cost per unit, $ 2,000 fixed...
-
An architectural design firm is faced with a decision as to which projects to bid on for the coming year. Ten projects are available for which they are qualified. The following table lists the profit...
-
Can a charged particle move through a magnetic field without experiencing any force? If so, how? If not, why not?
-
Review the chapters opening case and some of the sample documents related to it. How do you think Debra handled the various stakeholders? Offer at least two suggestions for what she might have done...
-
On September 16, 20X8, Toys R Us, the worlds largest toy seller, Announced strategic initiatives tore structure its business. The total cost of implement these initiatives yielded a charge of $508...
-
The following accounts are from last year's books at Sharp Manufacturing: \ table [ [ , Raw Materials ] , [ , Debit,Credit, ] , [ \ table [ [ Balance ] , [ ( a ) ] ] , 0 , ( b ) , 1 5 5 , 2 0 0 ] , [...
-
Determine Zi Zo, and Vo for the network of Fig. 8.76 if Vi = 20 mV. +20 V 2 kS2 82 /oss= 12 mA z, Z, Cs 610
-
What alkyne would you start with to prepare each of the following compounds by a hydroboration/oxidationreaction? (b) (a) CH CH-CCHCH3 -CH2CH CH
-
The pK a of acetone, CH 3 COCH 3 , is 19.3. Which of the following bases is strong enough to de-protonate acetone? (a) KOH (p K a of H 2 O = 15.7) (b) Na + C CH (p K a of C 2 H 2 = 25) (c) NaHCO 3...
-
The Baking Department at Rainbow Baker had a beginning work in process inventory of 6,000 cakes, completed 10,000 cakes, and had 5,000 cakes in ending work in process inventory in February:...
-
Gulf Shore Lawn and Garden Maintenance provides two general outdoor services: lawn maintenance and garden maintenance. The company charges customers $18.0 per hour for each type of service, but lawn...
-
Two level sections of an east highway (G=0) are to be connected. Currently, the two sections of highway are seperated by a 4000-ft (horizontal distance), 2% grade. The westernmost section of highway...
-
A solution contains 2 x 10-3 moles Ca2+/L and 3 x 10-4 moles Mg2+/L. Given the formation constants for CaEDTA2- and MgEDTA2- of 1010.6 and 108.7, respecively, calculate: 1) Concentration of MgEDTA2-...
-
The direct material (DM) price variance is $2,650 favorable and the DM usage variance is $3,000 unfavorable. The budgeted amount of DM for each unit of product is 2 lbs. to be purchased at the...
-
On January 1, 2023, AMI Corporation purchased the non-cash net assets of Oriole Ltd. for $8,399,900. Following is the statement of financial position of Oriole Ltd. from the company's year- end the...
-
Organization: Gaming floor Web site: www.gamingfloor.com Summary: This is an online resource for casino trade and industry news across different continents. a. Identify the countries in the Asia...
-
Given the table below, about how much force does the rocket engine exert on the 4.0 kg payload? Distance traveled with rocket engine firing (m) Payload final velocity (m/s) 500 320 490 310 1020 450...
-
Write balanced complete ionic and net ionic equations for each acidbase reaction. a. HBr(aq) + NaOH(aq) b. HF(aq) + NaOH(aq) C. HCHO(aq) + RbOH(aq)
-
Classify the following peptides as acidic, basic, or neutral. What is the net charge on each peptide at pH = 6? (a) Gly-Leu-Val (b) Leu-Trp-Lys-Gly-Lys (c) N-acetyl-Asp-Val-Ser-Arg-Arg (A-acetyl...
-
Classify each of the following pericyclic reactions as an electrocyclic, cycloaddition, or sig- matropic reaction. Give the curved-arrow notation for each reaction, and tell how many electrons are...
-
Show by a frontier orbital analysis that the [Aa + 2s] and [4s + 2a] modes of cycloaddition are not allowed.
-
Which of the following is a limitation of both return on investment and residual income? A. Favors large units. B. There is disincentive for high return on investment units to invest. C. Can lead to...
-
For anOld Country Links, Incorporated, produces sausages in three production departments Mixing , Casing and Curing, and Packaging. In the Mixing Department, meats are prepared, ground and mixed with...
-
A manufacturing firm uses a predetermined manufacturing overhead rate to allocate overhead to individual jobs, based on machine hours required. At the beginning of 2 0 1 9 , the firm expected to...

Study smarter with the SolutionInn App


