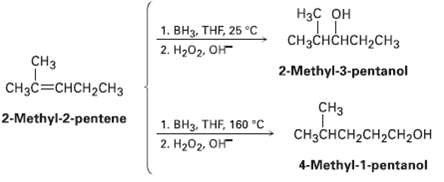
Hydroboration of 2-mnethyl-2-pentene at 25?C followed by oxidation with alkaline 11202 yields 2-methyl-3-pentanol, but hydroboration at 160?C
Question:
Hydroboration of 2-mnethyl-2-pentene at 25?C followed by oxidation with alkaline 11202 yields 2-methyl-3-pentanol, but hydroboration at 160?C followed by oxidation yields 4-methyl?1?pentanol. Suggest a mechanism.
Transcribed Image Text:
Нас он CHзснсHсH2сH3 1. ВНа, THF 25 °C 2. Hа02, Он CHз 2-Methyl-3-pentanol CH3C=CHCH2CH3 CH3 2-Methyl-2-pentene 1. BH3, THF, 160 "C CHзснсH2сH2CH2он 2. H202, OH 4-Methyl-1-pentanol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Hydroboration of 2methyl2pentene at 160C is reversible The ini...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Oxidation of an aldehyde yields a carboxylic acid: Draw the structures for the products of the following oxidation reactions. a. b. c. [ox] propanal 2,3-dimethylpentanal ox] 3-ethylbenzaldehyde>
-
Select any Company and get a copy of its annual report. Just write an outline of the company's annual report. The outline must contain the following: 1. Introduction 2. Background 3. Liquidity Ratios...
-
An abc-sequence balanced three-phase wye-connected source supplies power to a balanced wye-connected load. The line impedance per phase is 1 + j10, and the load impedance per phase is 20 + j20. If...
-
An ad for a cell phone service claims that its percent of "dropped calls" was significantly lower than that of its main competitor. In the fine print, the percents were given as 1.2 percent versus...
-
The following is the statement of financial position of WW Associates as at 31 December 2008: During 2009, the following transactions took place: 1 The owners withdrew equity in the form of cash of...
-
Certain liability and net worth items generally increase spontaneously with increases in sales. Put a check mark ( ) next to those items that typically increase spontaneously. Accounts payable...
-
How can firms best ensure that their code of business ethics ensure is read, understood, believed, remembered, and acted on, rather than ignored?
-
I have to do a financial assignment in Capital Markets. I had to download from Bloomberg the monthly Data for the period 2000-2015: 1. Monthly Price Data of several stocks 2. Price Data for the FTSE...
-
What are the pros and cons of organizing a plant within-a-plant?
-
Treatment of 4-penten-l-ol with aqueous Br2 yields a cyclic bromo ether rather than the expected bromohydrin. Suggest a mechanism, using curved arrows to show electronmovement. CH2B Br2, H20 %3...
-
We?ll see in the next chapter that alkynes undergo many of the same reactions that alkenes do. What product might you expect from each of the following reactions? 1 equiv Br2 CH3 (a) 2 equiv H2, Pd/C...
-
Fluid flows down a long cylindrical pipe of length b much larger than radius a, from a reservoir maintained at pressure P 0 (which connects to the pipe at x = 0) to a free end at large x, where the...
-
Spitfire Company makes and sells three products: A, B, and C. The following data relate to these products: A B Demand in units Selling price per unit 110 100 90 $180 $210 $195 Raw material costs per...
-
NCF & Partners (NCF) is a firm of CPAslocated in Whitby that has been in business for 20 years. NCF's revenue has declined steadily over the past few years. The partners are looking for ways...
-
Task 4.2Written report Describe how you will present the menu to customers, for example, folders, covers, boards or binding. Include details of colour schemes, pictures, icons, logos, symbols and...
-
The American company "Amazonian", leader in food distribution, is starting operations in Brazil. They just hired a group of new managers who will lead several branches of the company in different...
-
1; Assume you are in charge of fundraising for an organization on your campusa social fraternity or sorority, a business fraternity, or any other such organization. It is your job to identify a...
-
Explain why managerial accounting in the hospitality industry is different from managerial accounting used in other industries.
-
Using a graphing utility, graph y = cot -1 x.
-
The results of a molecular orbital calculation for H 2 O are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons...
-
Show how the stereochemistry of the products will differ (if at all) when the following enantiomerically pure epoxide is hydrolyzed under acidic and basic conditions. D,C
-
(a) From what Grignard reagent can 3-methl-l pentanol be prepared by reaction with ethylene oxide, then aqueous acid? (b) Give the structure of another epoxide and another higher-order curpate that...
-
Explain why all attempts to isolate trimethyloxonium iodide lead instead to methl iodide and dimethl ether.
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...
Dimensional Analysis And Scale Up In Chemical Engineering 1st Edition - ISBN: 3540541020 - Free Book

Study smarter with the SolutionInn App


