The results of a molecular orbital calculation for H 2 O are shown here. Examine each of
Question:
The results of a molecular orbital calculation for H2O are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons to the energy diagram. According to this energy diagram, is H2O stable? Explain.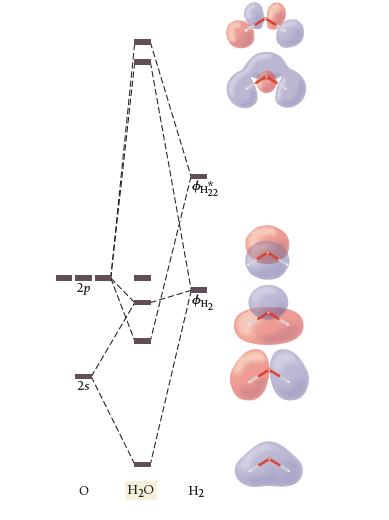
Transcribed Image Text:
I 2p 10H22 $112 O H₂O H₂ 80
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Classification of orbitals Bonding orbitals 1a1 2a1 1b2 Antibonding orbitals 3a1 1b1 2b...View the full answer

Answered By

ALBANUS MUTUKU
If you are looking for exceptional academic and non-academic work feel free to consider my expertise and you will not regret. I have enough experience working in the freelancing industry hence the unmistakable quality service delivery
4.70+
178+ Reviews
335+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The results of a molecular orbital calculation for NH 3 are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons...
-
The FEMO theory (Problem 11.14) of conjugated molecules is rather crude and better results are obtained with simple Huckel theory. (a) For a linear conjugated polyene with each of N carbon atoms...
-
Construct an approximate molecular orbital energy diagram for a hypothetical planar form of NH 3 . You may refer to Resource section 4 to determine the form of the appropriate orbitals on the central...
-
why people who are sophisticated but face borrowing constraint would increase consumption when they receive transfer payment?
-
Martin Company is considering the purchase of a new piece of equipment. Relevant information concerning the equipment follows: Purchase cost . . . . . . . . . . . . . . . . . . . . . $180,000 Annual...
-
Suppose you are an analyst for the Roanoke Software Consulting Company (RSCC), a large consulting firm with offices around the world. The company wants to build a new knowledge management system that...
-
Discuss the importance of good operational procedures and how such procedures might be established within an organization.
-
Deepa Dalal opened a free-standing radiology clinic. She had anticipated that the costs for the radiological tests would be primarily fixed, but she found that costs increased with the number of...
-
For Oriole Company, actual sales are $1,450,000, and break-even sales are $971,500. (a) Compute the margin of safety in dollars. Margin of safety $ LINK TO TEXT (b) Compute the margin of safety...
-
Market Corporation owns 100% of Subsidiary Corporation's stock. Market Corporation completely liquidates Subsidiary Corporation, receiving land with a $400,000 adjusted basis and a $500,000 FMV in...
-
The species NO 2 , NO 2 + , and NO 2 - in which N is the central atom have very different bond angles. Predict what these bond angles might be with respect to the ideal angles and justify your...
-
cis-2-Butene isomerizes to trans-2-butene via the reaction shown here. a. If isomerization requires breaking the p bond, what minimum energy is required for isomerization in J/mol? In J/molecule? b....
-
Perform the indicated operations assuming all numbers are approximate and round the answer appropriately. 0.6572(3.94)
-
Write out the form of the partial fraction decomposition of the function (see example). Do not determine the numerical values of the coefficients. x3 (a) x + 7x+6 9x+1 (b) (x + 1)3(x + 2) Submit...
-
You desire to make an 80% by weight vinyl acetate to 20% by weight styrene copolymer via free radical, emulsion polymerization. The r 1 and r 2 values for these monomers are 0.01 and 55,...
-
Q1)In a wheel and axle machine the diameters of the wheel and the axle are 450mm and 60mm respectively.The efficiency is 97%(0.97 per unit).When a body having a mass of 40kg is being lifted.Determine...
-
Smith & Chief Ltd. of Sydney, Australia, is a merchandising firm that is the sole distributor of a product that is increasing in popularity among Australian consumers. The company's income statements...
-
C. In lab, you measure the x & y components of a possible incompressible flow field as u = 2cxy; and where cand a are constants. v = c(a + x - y) 5. (04 pts) Short answer, what is necessary for the...
-
Draw structural formulas for the three possible isomers of C3H8O.
-
Solve each equation. x 3 - 6x 2 = -8x
-
The bar has a cross-sectional area of 400(10 6 ) m 2 . If it is subjected to a triangular axial distributed loading along its length which is 0 at x = 0 and 9 kN/m at x = 1.5 m, and to two...
-
The bar has a cross-sectional area of 400(10 6 ) m 2 . If it is subjected to a uniform axial distributed loading along its length of 9 kN/m, and to two concentrated loads as shown, determine the...
-
The two members used in the construction of an aircraft fuselage are joined together using a 30° fish-mouth weld. Determine the average normal and average shear stress on the plane of each weld....
-
A BOND IS SELLING AT 777 DOLLAR AND COUPON RATE IS 7% WHAT IS THE CURRENT YIELD ROUND THE NUMBER TO 2 DECIMALS (0.00)
-
ABC Corporation gathered the following information relating to its inventories: (A) Inventories per physical count P3,000,000; (B) Inventories consigned to ABC included in the count P100,000; (C)...
-
Documenti Word Layout References 1.ailing: Rei View Help Tell me you want to do 53. You are to read the attached notes and develop two items that will be added to the answer sheet under question 74...

Study smarter with the SolutionInn App


