cis-2-Butene isomerizes to trans-2-butene via the reaction shown here. a. If isomerization requires breaking the p bond,
Question:
cis-2-Butene isomerizes to trans-2-butene via the reaction shown here.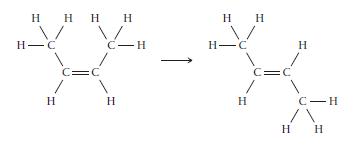
a. If isomerization requires breaking the p bond, what minimum energy is required for isomerization in J/mol? In J/molecule?
b. If the energy for isomerization came from light, what minimum frequency of light would be required? In what portion of the electromagnetic spectrum does this frequency lie?
Transcribed Image Text:
H Н Н Н Н н н H H-C H =C с-н Н Н H H-C н- 1 Н Н Н C Н C-H н н нн
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a To calculate the minimum energy required for isomerizationwe need to know the bond energy of the b...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction shown here was performed with an iridium catalyst, both in supercritical CO2 (scCO2) and in the chlorinated solvent CH2Cl2. The kinetic data for the reaction in both solvents are plotted...
-
The energy of a vibrating molecule is quantized much like the energy of an electron in the hydrogen atom. The energy levels of a vibrating molecule are given by the equation: where n is a quantum...
-
The first LEDs were made from GaAs, which has a band gap of 1.43 eV. What wavelength of light would be emitted from an LED made from GaAs? What region of the electromagnetic spectrum does this light...
-
Gems Co. uses the indirect method to prepare its statement of cash flows. The following comparative statement of financial position for 2021 and 2022 are presented: At December 31 2022 2021 Property,...
-
Worldwide Travel Service has made an investment in certain equipment that cost the company $307,100. The equipment is expected to generate cash inflows of $50,000 each year. Required: How many years...
-
Consumer electronics is a very competitive business. What might be the success story of the year one year is a forgotten item two years later. Rapid product commoditization makes the consumer...
-
Describe the different types and categories of controls that exist in most large organizations and explain what could go wrong, even where controls are meant to be in place.
-
a. Discuss the factors that influence an antimicrobial agents ability to completely remove microbial contaminants from dentures used on a daily basis. b. Improper cleaning of endoscopes has led to...
-
Oranter Points Tarnet Deliverable Length See segment details View before Looking for tutoring Get Saching Assignment Details Assignment Description AM Vegas started a business called astees. The...
-
The finance director of RM plc is considering several investment projects and has collected the following information about them. Projects D and E are mutually exclusive. The capital available for...
-
The results of a molecular orbital calculation for H 2 O are shown here. Examine each of the orbitals and classify them as bonding, antibonding, or nonbonding. Assign the correct number of electrons...
-
In VSEPR theory, which uses the Lewis model to determine molecular geometry, the trend of decreasing bond angles in CH 4 , NH 3 , and H 2 O is accounted for by the greater repulsion of lone pair...
-
Nitrogen forms several compounds with oxygen, including nitrogen dioxide and dinitrogen monoxide. Nitrogen dioxide contains 2.28 g oxygen to every 1.00 g nitrogen, while dinitrogen monoxide contains...
-
Year 5% 6% 4 3.546 3.465 5 7% 3.387 3.312 4.329 4.212 4.100 8% 3.993 5.076 4.917 4.767 4.623 Present Value of an Annuity of $1 at Compound Interest 9% 10% 11% 12% 13% 14% 15% 3.240 3.170 3.102 3.037...
-
2. Determine the overturning stability of the cantilever retaining wall shown. The equivalent fluid density is 5.5 kN/m, soil density is 18 kN/m, and the concrete weighs 23.5 kN/m. (5 pts) 2 m 2 m 2...
-
A. For a certain two-dimensional, incompressible flow field the velocity component in the y direction is given by v = 3xy + xy 1. (05 pts) Short answer, what is the condition for this flow field to...
-
Cho0se a hazardous material to cr3ate a pr3sentation on (i.e. sulfuric acid, explosives, used needles, there are many types of hazardous materials) Cr3ate a presentation (P0werPoint, Open0ffice...
-
If det [a b] = c d 2 -2 0 a. det c+1 -1 2a d-2 2 2b -2 calculate:
-
Using Table 1.3, determine what charge the ion will carry when each of the following elements reacts to form an ionic compound: Al, Li, S, and O. Table 1.3 Table 1.3 Valence Electrons of the First 18...
-
Solve each equation or inequality. |6x8-4 = 0
-
The 2-Mg concrete pipe has a center of mass at point G. If it is suspended from cables AB and AC, determine the average normal stress in the cables. The diameters of AB and AC are 12 mm and 10 mm,...
-
The 2-Mg concrete pipe has a center of mass at point G. If it is suspended from cables AB and AC, determine the diameter of cable AB so that the average normal stress in this cable is the same as in...
-
The pier is made of material having a specific weight g. If it has a square cross section, determine its width w as a function of z so that the average normal stress in the pier remains constant. The...
-
Penske Ltd has a standard deviation of returns of 18% and a correlation with the market portfolio of 0.8. The market portfolios expected return is 14%, its standard deviation of returns is 12%, and...
-
In an insurance policy identification of the named insured is important in terms of determining who is the insured. In the body of the policy the names insured oftentimes is referred to as "you"....
-
During the year Salaries payable decreased by $6,000. If Salary expense amounted to $160,000 for the year, the cash paid to employees is: Select one: a. $160,000. b. $172,000. c. $166,000. d....

Study smarter with the SolutionInn App


