Identify all the acidic hydrogen?s (pKa (b) (c) (a) CHCH2H3 CH H-CH23H C02CH (f) COCI (d) (e)
Question:
Identify all the acidic hydrogen?s (pKa
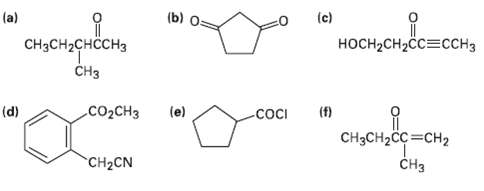
Transcribed Image Text:
(b) (c) (a) CHзCH2снссH3 CHз носH-CH2сс3ссHз C02CHЗ (f) COCI (d) (e) CHзCH2CC 3CH2 CHз CH2CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (18 reviews)
Acidic hydrogens are bold The most acidic hydrogens are the t...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Hydrogen cyanide (HCN) has a pKa of 9.1. What is its Ka? Is HCN a strong or a weak acid?
-
Identify which of the following compounds is more acidic and explain your choice.
-
Identify the more acidic compound in each of the following pairs: (a) CF3CH2CO2H or CF3CH2CH2CO2H (b) CH3CH2CH2CO2H or CH3CPCCO2H (c) (d) (e) (f) (g) CO2H CO2H or CO,H CO2H o F- COH CO.H -CO-H or...
-
A general ledger trial balance at June 30, 2011, for Millar City is as follows: Millar City uses a purchases basis in accounting for supplies. Open encumbrances are considered constrained by the...
-
How are direct-mail sales messages and e-mail sales messages similar, and how are they different?
-
1. If the SDR were used, would a viable fixed-rate regime be possible or not? 2. Given the inclusion of the SDR, outline the pro and con arguments for a fixed-rate regime. The global debate issue...
-
*Show that the equation-by-equation least-squares estimator Bb X0 X (1 X0 Y is the maximum-likelihood estimator of the regression coefficients B in the multivariate general linear model Y XB E,...
-
On January 2, 2015, the Matthews Band acquires sound equipment for concert performances at a cost of $65,800. The band estimates it will use this equipment for four years, during which time it...
-
Question 2 The balance sheet of Nisha Ltd as at 31 st March 2017 LIABILITIES AMOUNT(N$) ASSETS AMOUNT(N$) -Equity share of N$10 each F.P -12% preference share of N$100 each F.p -10%Debenture...
-
Schank Marketing Research has just signed contracts to conduct studies for four clients. At present, three project managers are free for assignment to the tasks. Although all are capable of handling...
-
For a given a hydrogen atom to be acidic, the C?H bond must be parallel to the p orbital?s of the C=O bond (that is, perpendicular to the plane of the adjacent carbonyl group). Identify the most...
-
Rank the following compounds in order of increasingacidity: (a) CH3CH2CO2H (b) CH3CH2OH (c) (CH3CH2)2NH (d) CH3COCH3 (e) (f) CCI3CO2H CCH-CCH3
-
Classify each of the following actions as either being associated with the financial accounting information system (FS) or the cost management information system (CMS): a. Determining ways to...
-
What are the major immediate concerns for the HR manager in Austral Group SAA when merging two different organizational cultures - in this case, Peruvian and Norwegian cultures?
-
Explain the relation between the corporate, business and functional strategies. Please produce an in-depth explanation.
-
Consider the problem of terrorism during Radical Reconstruction. If you had been an adviser to the President, how would you propose to deal with the problem? Give a minimum of TWO examples and fully...
-
describe at least one element of an Airport Master Plan. Discuss the importance of this element and how it fits into the overall Airport Master Plan document to include its processes and objectives.
-
It is suggested that Wikipedia has replaced the hardback encyclopedia books, such Encyclopedia Brittanica. What other ways do you foresee technology changing businesses that have been around for...
-
What are the advantages of using a Strategic alliance when operating in a new country? AppendixLO1
-
Does log 81 (2401) = log 3 (7)? Verify the claim algebraically.
-
Use MO theory to predict the bond order in H 2 . Is the H 2 bond a stronger or weaker bond than the H 2 bond?
-
Complete the following Pd(0)-catalyzed Suzuki reactions by giving the coupling product. Include the stereochemistry of the products. BOH CH3 CH3O
-
Give two different pairs of starting materials that could be used to prepare the following compound by a Suzuki coupling. CH3 Ph C-C
-
Draw structures analogous to those in Eqs. 18.59a-d for the catalytic intermediates formed in the conversion of 1, 7-octadiene to cyclohexene and ethylene catalyzed by the G2 catalyst. Eqs. 18.59a-d...
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


