In 1932, A. A. Levine and A. G. Cole studied the ozonolysis of o-xylene and isolated three
Question:
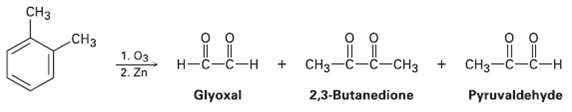
In 1932, A. A. Levine and A. G. Cole studied the ozonolysis of o-xylene and isolated three products: glyoxal, 2, 3-butanedione, and pyruvaldehyde: In what ratio would you expect the three products to be formed if o-xylene is a resonance hybrid of two structures? The actual ratio found was 3 parts glyoxal, 1 part 2, 3-butanedione, and 2 parts pyruvaldehyde. What conclusions can you draw about the structure ofo-xylene?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





