In step 2 of the citric acid cycle (Figure), cis-aconitate reacts with water to give (2R, 3S)-isocitrate.
Question:
In step 2 of the citric acid cycle (Figure), cis-aconitate reacts with water to give (2R, 3S)-isocitrate. Does ?OH add from the Re face of the double bond or from the Si face? What about ?H? Does the addition of water occur with syn or anti geometry?
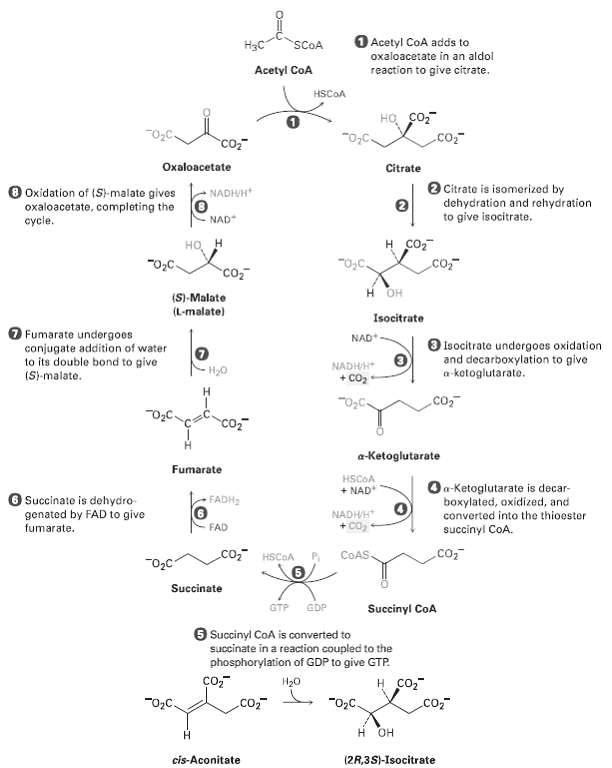
O Acetyl CoA adds to H3C SCOA oxaloacetate in an aldol Acetyl CoA reaction to give citrate. HSCOA HO co, .co co2 Oxaloacetate Citrate O Citrate is isomerized by dehydration and rehydration to give isocitrate. O Oxidation of (St-malate gives oxaloacotate, completing the cycie. NADHVH NAD н сот но "O,C. co, "0,C. Co H OH (S)-Malate (L-malate) Isocitrate O Fumarate undergoes conjugate addition of water to its double bond to give (S-malate. NAD* O lsocitrate undorgoes oxidation and decarboxylation to give a-kotoglutarato. NADHH H20 + co2 H. co, "O2C. co, a-Ketoglutarate Fumarate HSCOA Oa-Ketoglutarate is decar- boxylated, oxidized, and converted into the thioester + NAD O Succinate is dehydro genated by FAD to give fumarate. FADH2 NADHVH + Co2 FAD succinyl CoA. CO HSCOA .co, COAS, Succinate GTP GDP Succinyl CoA 6 Succinyl CoA is converted to succinate in a reaction coupled to the phosphorylation of GDP to give GTP. H20 co, "02C. "02C. .co, H OH Н cis-Aconitate (2R,3S)-Isocitrate
Step by Step Answer:
Addition of OH The top face is the Re face and OH adds from this fac...View the full answer



Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Organic Chemistry questions
-
Write mechanisms for step 2 of the citric acid cycle, the dehydration of citrate and the addition of water to aconitate.
-
The citric acid cycle can be divided into two phases with respect to the oxidation of acetyl- CoA. Describe each phase and write its balanced equation.
-
The first step in the citric acid cycle is reaction of oxaloacetate with acetyl CoA to give citrate. Propose a mechanism, using acid or base catalysis asneeded. "O2C. 02C H .co2 CO SCOA Citrate...
-
Explain why the coffee shop manager should measure elasticity using the mid-point method in his calculations.
-
Financial models normally are said to be in balance when total assets equal total liabilities plus shareholders' equity on the balance sheet. How are financial models often forced to balance...
-
After collecting, compiling, and analyzing the data, what conclusions and recommendations would Jeff be justified in making in his report to Marcus?
-
False negative rate.
-
Consider a sample with data values of 10, 20, 12, 17, and 16. Compute the z-score for each of the five observations.
-
Analyzing and Computing Average Issue Price and Treasury Stock Cost Assume this is the stockholders' equity section from the Campbell Soup Company balance sheet. Shareholders' Equity (millions,...
-
Stephens Supply, Inc. (SSI) has a market value of equity of $70 million and an equity beta of 1.6. SSI has cash of $9 million and debt of $21 million. Assume the risk-free rate is 3% and the market...
-
Write a mechanism for the conversion of -ketoglutarate to succinyl CoA in step 4 of the citric acid cycle(Figure). O Acetyl CoA adds to oxaloacetate in an aldol H3C SCOA Acetyl CoA reaction to give...
-
The primary fate of acetyl CoA under normal metabolic conditions is degradation in the citric acid cycle to yield CO2. When the body is stressed by prolonged starvation, however, acetyl CoA is...
-
Describe each of the three categories of diagrams.
-
What is the length of the partial wavelength for electromagnetic energy with a frequency of 15 MHz and a phase shift of 263 degrees (in meters)?
-
Let y = f(x) be a function that is differentiable at all real numbers. Suppose f has the form f(x) = {; [cx +6 4+2c for x 1 for x > 1. where b and c are some constants. What is the value of the...
-
Consider the sequence (n) defined by xn = (a) Show that 0In - n! nn (b) Use the result of part (a) and the Squeeze theorem to show that In 0 and n o.
-
Officials of Gwinnett County, one of the fastest growing counties in the country, are looking for ways to expand their sewer system. They are considering two alternative sewer designs. All annual...
-
On August 1, 20Y7, Rafael Masey established Planet Realty, which completed the following transactions during the month: Rafael Masey transferred cash from a personal bank account to an account to be...
-
What are the steps involved in win win problem solving?
-
On January 1, 2017, McIlroy, Inc., acquired a 60 percent interest in the common stock of Stinson, Inc., for $340,200. Stinson's book value on that date consisted of common stock of $100,000 and...
-
Redo Problem 14.13 using Aspen Plus. Problem 14.13 Equal amounts of pure nitrogen and pure oxygen, each at 3000 K and 1 bar, are continuously fed into a chemical reactor, and the reactor effluent,...
-
Suggest a sequence of reactions suitable for preparing each of the following compounds from the indicated starting material. You may use any necessary organic or inorganic reagents. (a) 1-Propanol...
-
Two different compounds having the molecular formula C8H15Br are formed when 1,6- dimethylcyclohexene reacts with hydrogen bromide in the dark and in the absence of peroxides. The same two compounds...
-
On catalytic hydrogenation over a rhodium catalyst, the compound shown gave a mixture containing cis-1-tert-butyl-4-methylcyclohexane (88%) and trans-1-tert-butyl-4 methylcyclohexane (12%). (a) What...
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


