Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an
Question:
Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an orbital picture of indole.
(a) How many ? electrons does indole have?
(b) What is the electronic relationship of indole to naphthalene?
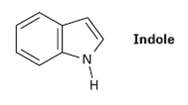
Transcribed Image Text:
Indole
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)
H H H H Indole Indole lik...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How many electrons does an Se atom have to gain to have a complete octet in its valence shell?
-
How many electrons does an N atom have to gain to have a complete octet in its valence shell?
-
How many electrons does a Ba atom have to lose to have a complete octet in its valence shell?
-
Which of the following would be a reason China would place a tariff on Canadian lumber? A. China is trying to protect its domestic lumber industry. B. China is seeking to lower the cost of lumber for...
-
How does FedEx handle customer service issues in a way that is different than most?
-
Calculate the amount financed, the monthly payment (assuming that monthly payments stay the same throughout the term of the loan), the total interest paid, and an amortization schedule. For the...
-
Compute business owners investment rates of return.
-
Income statement information for Einsworth Corporation is provided below. Sales ..........$1,200,000 Cost of goods sold..... 780,000 Gross profit........ 420,000 Prepare a vertical analysis of the...
-
Help me Over here Please Guys 1. In order to provide more accountability it is important to set goals and to break them down. True False 2. Which of these prospects have been to your site before,...
-
Pentalene is a most elusive molecule and has never been isolated. The pentalene dianion, however, is well known and quite stable. Explain. 12- Pentalene Pentalene dianion
-
Ribavirin, an antiviral agent used against hepatitis C and viral pneumonia, contains a 1, 2, 4-triazole ring. Why is the ringaromatic? 1,2,4-Triazole ring N- NH2 N-N Ribavirin OH
-
A spherical snowball is melting in such a way that diameter is decreasing at rate of 0.2 cm/min. At what rate is the volume of the snowball decreasing when the diameter is 10 cm.
-
Prove (11.32) . E (Yi,k | Zi = 0, = e) = E (Yi,k | i = 1, = e) = E (Yi,k | Ti = e), k = 1,2. (11.32)
-
University Medical Center needs to move from its existing facility to a new and larger facility five miles away from its current location. Due to construction delays, however, much of the new...
-
Calculate the base value or lump sum for each of the single and married filing jointly 2016 brackets given in Table 6.4. Table 6.4 ITABLE 6.4 Corporate Income Brackets and Tax Rates, 2015 Taxable...
-
Show that staged column diameter is proportional to (feed rate) \({ }^{1 / 2}\) and to \((1+\mathrm{L} / \mathrm{D})^{1 / 2}\).
-
An atmospheric column with 25 real stages is operating with a pressure drop of 0.6 in. of water per stage. Assume pressure drop in the condenser and the reboiler is \(1.2 \mathrm{in}\). of water...
-
explain and deal with the particular difficulties which may be met with in preparing such statements;
-
Suppose you are comparing just two means. Among the possible statistics you could use is the difference in means, the MAD, or the max min (the difference between the largest mean and the smallest...
-
Which statement does NOT generally apply to a chemical reaction in dynamic equilibrium? (a) The rates of the forward and reverse reactions are equal. (b) The concentrations of the reactants and...
-
Sometimes chemists need the unnatural D enantiomer of an amino acid, often as part of a drug or an insecticide. Most L-amino acids are isolated from proteins, but the D-amino acids are rarely found...
-
Although tryptophan contains a heterocyclic amine, it is considered a neutral amino acid. Explain why the indole nitrogen of tryptophan is more weakly basic than one of the imidazole nitrogens of...
-
Draw the electrophoretic separation of Ala, Lys, and Asp at pH 9.7.
-
DISCUSSION ACTIVITY All jurisdictions have legislation protecting seniority and benefits for qualified employees who are members of the Canadian Forces Reserves and who are deployed for active...
-
Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Firm J has net income of $90,160, sales of $980,000, and average total assets of $490,000. Required:...
-
Read Chapter 5 and the Tyco case and identify some of the signals of the misuse of acquisitions or merger reserves. How could these signals have helped the users of Tyco's financial statements...

Study smarter with the SolutionInn App


