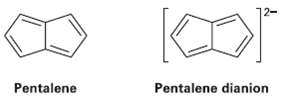
Pentalene is a most elusive molecule and has never been isolated. The pentalene dianion, however, is well
Question:
Pentalene is a most elusive molecule and has never been isolated. The pentalene dianion, however, is well known and quite stable. Explain.
Transcribed Image Text:
12- Pentalene Pentalene dianion
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
Pentalene has eight elect...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The HCI molecule is quite well described by the Morse potential with De = 5.33 eV, V = 2989.7 cm-1, and XV = 52.05 cm-I. Assuming that the potential is unchanged on deuteration, predict the...
-
A South Korean research group has isolated and synthesized "daumone," the pheromone that induces hibernation in Caenorhabditis elegans worms when food becomes scarce, thus extending their life span...
-
The resonance form shown is not the most stable one for the compound indicated. Write the most stable resonance form.
-
Harvold Company's quality cost report is to be based on the following data: Test and inspection of incoming materials. $71,000 Supplies used in testing and inspection . Re-entering data because of...
-
Think of a positive customer experience. What are the behavioral success factors that made the encounter so successful?
-
In some ordering problems, like the one for Sams Bookstore, whenever demand exceeds existing inventory, the excess demand is not lost but is filled by expedited ordersat a premium cost to the...
-
Identify advantages and disadvantages of capital financing alternatives such as debt versus equity financing and lease versus buy decisions.
-
During the current month, the following errors occurred in recording transactions in the purchases journal or in posting from it. a. An invoice for $1,875 of supplies from Kelly Co. was recorded as...
-
Bullseye Company manufactures dartboards. Its standard cost information follows: Standard Quantity 2.5 sq. ft. 1 hr. Standard Price (Rate) $ 2.00 per sq. ft. $14.00 per hr. Standard Unit Cost $ 5.00...
-
Assume the same information for TelMark as in Exercise 11-24. A member of the data science team points out that overfitting is often an issue with decision trees. To avoid this issue, he suggests...
-
Calicene, like azulene (Problem 15.17), has an unusually large dipole moment for a hydrocarbon. Explain, using resonancestructures. Calicene
-
Indole is an aromatic heterocycle that has a benzene ring fused to a pyrrole ring. Draw an orbital picture of indole. (a) How many ? electrons does indole have? (b) What is the electronic...
-
Interestingly, there have been several studies using cadavers to determine the moments of inertia of human body parts, information that is important in biomechanics. In one study, the center of mass...
-
Customers arrive at a ferry ticket office at the rate of 14 per hour on Monday morn- ings. This can be described by a Poisson distribution. Selling the tickets and pro- viding general information...
-
Glen County manages a waste-to-energy facility that burns 2,000 tons of trash per day and generates over \($20\) million in electricity annually while costing state and local taxpayers \($24\)...
-
Carry out a full decision analysis for Classical Reproductions Ltd, using the following information: Calculation of expected profit with perfect information Prior probabilities for the various events...
-
T and B lymphocytes are normal components of the immune system, but in multiple sclerosis they become autoreactive and attack the central nervous system. What triggers the autoimmune process? One...
-
Prove (11.32) . E (Yi,k | Zi = 0, = e) = E (Yi,k | i = 1, = e) = E (Yi,k | Ti = e), k = 1,2. (11.32)
-
explain the particular needs for financial statements for clubs, societies and charities;
-
The Cholesterol Level data sets give cholesterol levels of heart attack patients. Cholesterol measures are taken 2, 4, and 14 days aft er a patient has suffered a heart attack. Is there a significant...
-
Consider the chemical equation and equilibrium constant for the synthesis of ammonia at 25 C: Calculate the equilibrium constant for the following reaction at 25 C: N(g) + 3 H(g) = 2 NH3(8) K = 5.6 X...
-
Glutathione (GSH) is a tripeptide that serves as a mild reducing agent to detoxify peroxides and maintain the cysteine residues of hemoglobin and other red blood cell proteins in the reduced state....
-
Complete hydrolysis of an unknown basic decapeptide gives Gly, Ala, Leu, Ile, Phe, Tyr, Glu, Arg, Lys, and Ser. Terminal residue analysis shows that the N terminus is Ala and the C terminus is Ile....
-
There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester. (a) Explain why an...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


