Lead experiences corrosion in an acid solution according to the reaction The rates of both oxidation and
Question:
Lead experiences corrosion in an acid solution according to the reaction
The rates of both oxidation and reduction half-reactions are controlled by activation polarization.
(a) Compute the rate of oxidation of Pb (in mol/cm2-s) given the following activation polarization data:
(b) Compute the value of the corrosionpotential.
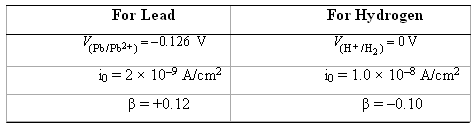
Transcribed Image Text:
For Lead P/P62+) =-0.126 V For Hydrogen '(H+ /H2) = OV i0 = 2 x 10-9 A/cm? B= +0.12 i0 = 1.0 x 10-8 A/cm2 B=-0.10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (19 reviews)
a This portion of the problem asks that we compute the rate of oxidation for Pb given that both the ...View the full answer

Answered By

Niala Orodi
I am a competent and an experienced writer with impeccable research and analytical skills. I am capable of producing quality content promptly. My core specialty includes health and medical sciences, but I can competently handle a vast majority of disciplines.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Materials Science and Engineering An Integrated Approach
ISBN: 978-1118061602
4th Edition
Authors: David G. Rethwisch
Question Posted:
Students also viewed these Materials Science Engineering questions
-
Lead experiences corrosion in an acid solution according to the reaction Pb + 2H+ Pb2+ + H2 The rates of both oxidation and reduction half-reactions are controlled by activation polarization. (a)...
-
Tertiary butyl chloride reacts in basic solution according to the equation The accepted mechanism for this reaction is What should be the rate law for this reaction? (CH3);CCI + OH (CH3),COH + CI
-
A solution is prepared from 0.150 mol of formic acid and enough water to make 0.425 L of solution. a. Determine the concentrations of H3O+ and HCOO in this solution. b. Determine the H3O+...
-
Selected answer is incorrect During substantive procedures, performing analytical procedures satisfies which primary audit objective: Cutoff Accuracy Existence Completeness 2 answers
-
Reveiw the paper "Managing Networks in the Age of Cloud, SDN, and Big Data: Network Management Megatrends 2014". Discuss at least two network management concepts you learned from the paper.
-
Using your example of audit sampling in the answer to R10.1, what items make up the population? What items are subject to being sampled? When the sample is complete, is the auditor drawing a...
-
Compute the present value of a proposed capital expenditure, using the "present value of an annuity of $1" table. (Obj. 5). Domino Manufacturing Corporation has prepared a schedule showing expected...
-
On November 1, 2016, Campbell Corporation management decided to discontinue operation of its Rocketeer Division and approved a formal plan to dispose of the division. Campbell is a successful...
-
Acme Alarm Systems Andrew Carter, the CEO of Acme Alarm Systems, could not hide his irritation from Becky Garcia over the proposed buyout of Internet Security, Inc. Becky, a recent graduate from the...
-
On January 1, 2024, LLB Industries borrowed $200,000 from Trust Bank by issuing a two-year, 10% note, with interest payable quarterly. LLB entered into a two-year interest rate swap agreement on...
-
(a) Describe the phenomenon of dynamic equilibrium as it applies to oxidation and reduction electrochemical reactions. (b) What is the exchange current density?
-
The corrosion rate is to be determined for some divalent metal M in a solution containing hydrogen ions. The following corrosion data are known about the metal and solution: (a) Assuming that...
-
KneeMan Markup Company has total debt obligations with book and market values equal to $30 million and $28 million, respectively. It also has total equity with book and market values equal to $20...
-
The transmitted energy expands out into space as it propagates at 3 GHz between the transmitter and the receiver over 30 km distance. Calculate the free space loss using a suitable formula and any...
-
What is the company featured in this episode of Undercover Boss? List 3 good professional activities that the CEO/president learned about their company by going undercover? List areas of the...
-
Assume there is a national lottery in the winning ticket is worth $10 million one winning ticket will be selected if there are 225 million tickets sold. What is the chance that a buyer of one ticket...
-
Description: Reference: Basu Thakur. (2015). PostcolonialTheory and Avatar (pp. 85-150,157-172). Bloomsbury PublishingUSAPre-Peer Paper Review for the Postcolonial Application Paper 1: Collecting...
-
NOT ASKING THE ACTUAL SHEAR STRESS. Please READ! Derive the shear stress distributed equation over the cross-section. Derive the equation and plot. 15 15 30 15 15 120 -90 20 0.5 m 72 kN 20 20 40 40...
-
Assume that a trial balance is prepared with an account balance of $18,950 listed as $18,590 and an account balance of $7,200 listed as $720. Identify the trans position and the slide. AppendixLO1
-
For the following exercises, find the inverse of the function and graph both the function and its inverse. f(x) = 4 x 2 , x 0
-
Write a query to display the current salary for each employee in department 300. Assume that only current employees are kept in the system, and therefore the most current salary for each employee is...
-
Why is speed synchronization of the various rolls so vitally important in a continuous or multistand rolling mill?
-
Explain how hot-rolled products can have directional properties and residual stresses.
-
Discuss the problems in maintaining uniform thickness in a rolled product and some of the associated defects.
-
Which of the following statements regarding traditional cost accounting systems is false? a. Products are often over or under cost in traditional cost accounting systems. b. Most traditional cost...
-
Bart is a college student. Since his plan is to get a job immediately after graduation, he determines that he will need about $250,000 in life insurance to provide for his future wife and children...
-
Reporting Financial Statement Effects of Bond Transactions (please show me how you got the answers) Lundholm, Inc., which reports financial statements each December 31, is authorized to issue...

Study smarter with the SolutionInn App


