Long-range coupling between protons more than two carbon atoms apart is sometimes observed when ? bonds intervene.
Question:
Long-range coupling between protons more than two carbon atoms apart is sometimes observed when ? bonds intervene. An example is found in 1-methoxy-1-buten-3-yne. Not only does the acetylenic proton, Ha, couple with the vinylic proton Hb, it also couples with the vinylic proton HC, four carbon atoms away. The data are: Construct tree diagrams that account for the observed splitting patterns of ?Ha? Hb, and HC
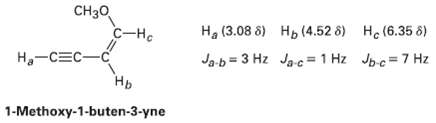
Transcribed Image Text:
CH30 С-на Ha (3.08 8) H, (4.52 8) He (6.35 8) Jpc=7 Hz Ja-b = 3 Hz Ja-c = 1 Hz Ha-CEC-C Нь 1-Methoxy-1-buten-3-yne
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Proton a 3088 Jah 3 Hz ...View the full answer

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Using compounds that possess no more than two carbon atoms, propose a plausible synthesis for the following compound.
-
Using any compounds that have no more than two carbon atoms, identify a method for preparing each of the following compounds: a. b. c. d.
-
HA and HB are both weak acids although HB is the stronger of the two. Will it take a larger volume of a 0.10 M NaOH solution to neutralize 50.0 mL of 0.10 M HB than would be needed to neutralize 50.0...
-
1. What trade- offs are involved in deciding to have a single large, centrally located facility instead of several smaller, dispersed facilities? 2. Who needs to be involved in facility location...
-
Explain why learning reduces the effective marginal cost of production. If firms set prices in proportion to their marginal costs, as suggested by the Economics Primer, how can learning firms ever...
-
Let A and B be events with P(A) = 0.3 and P(A B) = 0.7. a. For what value of P(B) will A and B be mutually exclusive? b. For what value of P(B) will A and B be independent?
-
In Question 9, would it make any difference if Rousseau had positive proof that a fraud had been perpetrated? nu6
-
The physical distribution channel of a major food manufacturer consists of plant stocks from which regional warehouses are restocked. These regional warehouses in turn supply the field warehouses...
-
Fergus Inc. has sales of $48.500 costs of $20,200, depreciation expense of $3,700, and Interest expense of $3,200. If the tax rate is 35%, what is the operating cash flow, or OCF? (Omit $ sign in...
-
Ewing Natural Gas is a large energy company with headquarters in Dallas, Texas. The company offers a wide variety of energy products and has annual revenues of approximately $50 billion. Because of...
-
Propose structures for the two compounds whose 1 H NMR spectra are shown. (a) C 4 H 9 Br (b) C 4 C 8 Cl 2 TMS O ppm 10 6. Chemical shift (8) 5 3 TMS O ppm 10 8. 6. Chemical shift (8) Intensity...
-
Assign as many of the resonances as you can to specific carbon atoms in the 13C NMR spectrum of ethylbenzoate. CH-CH TMS 100 Chemical shift (8) 200 180 140 20 0 ppm 120 40 160 60 Intensity
-
Data for Amsterdam Company are presented in BE8-5. Compute the April 30 inventory and the April cost of goods sold using the LIFO method. Data From BE 8-5: Amsterdam Company uses a periodic inventory...
-
Machine cost = $15,000; life = 8 years; salvage value = $3,000. What minimum cash return would an investor demand annually from the operation of this machine if he desires interest annually at the...
-
Write a program that prompts for the student's name, the number of exams, the exam score of each exam, and display the letter grade for the student. Read the entire problem description before coding....
-
Considering only the vertical stabilizer and rudder, explain the aerodynamic forces and moments that are created. You must include at least applicable airfoil terminology, description of force...
-
part. Review A bicycle wheel is rotating at 47 rpm when the cyclist begins to pedal harder, giving the wheel a constant angular acceleration of 0.44 rad/s. Part B How many revolutions does the wheel...
-
Suppose the number of students who register for a certain class each semester can be modeled by a Poisson distribution with average 10. Suppose further that each student passes the class with...
-
How do we measure concepts?
-
Suppose that fraction used = / 1.0 + 0.1Mt. for some parameter 1. Write the discrete-time dynamical system and solve for the equilibrium. Sketch a graph of the equilibrium as a function of ....
-
A KCl solution containing 42 g of KCl per 100.0 g of water is cooled from 60 C to 0 C. What happens during cooling?
-
Using the examples in Table 15-2 to guide you, match four of the following UV absorption maxima (λmax) with the corresponding compounds: (1) 232 nm; (2) 256 nm; (3) 273 nm; (4) 292 nm;...
-
Phenolphthalein is an acid-base indicator that is colorless below pH 8 and red above pH 8. Explain briefly why the first structure is colorless and the second structure is colored. HOT colorless red...
-
Classify the following dienes and polyenes as isolated, conjugated, cumulated, or some combination of these classifications. (a) cycloocta-1, 4-diene (b) cycloocta-1, 3-diene (c) cyclodeca-1, 2-diene...
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...

Study smarter with the SolutionInn App


