Assign as many of the resonances as you can to specific carbon atoms in the 13C NMR
Question:
Assign as many of the resonances as you can to specific carbon atoms in the 13C NMR spectrum of ethylbenzoate.
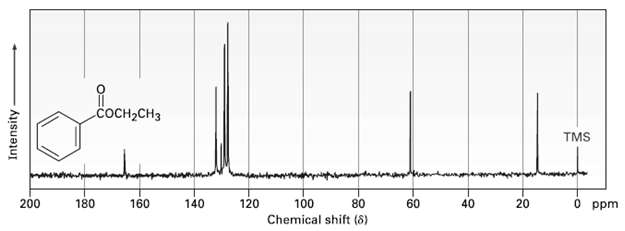
Transcribed Image Text:
соCH-CHз TMS 100 Chemical shift (8) 200 180 140 20 0 ppm 120 40 160 60 Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (18 reviews)
0 5 10 6 4 5 OO 2 ...View the full answer

Answered By

ZIPPORAH KISIO LUNGI
I have worked on several other sites for more than five years, and I always handle clients work with due diligence and professionalism. Am versed with adequate experience in the fields mentioned above in which have delivered quality papers in research, thesis, essays, blog articles, and so forth.
I have gained extensive experience in assisting students to acquire top grades in biological, business and IT papers. Notwithstanding that, I have 7+ years of experience in corporate world software design and development.
5.00+
194+ Reviews
341+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
3-Methyl-2-butanol has five signals in its 13C NMR spectrum at 17.90, 18.15, 20.00, 35.05, and 72.75 ?. Why are the two methyl groups attached to C3 nonequivalent? Making a molecular model should be...
-
What changes would you expect in the 13C NMR spectrum of 1-bromopropane upon cooling the compound to very low temperature?
-
Assign the resonances in the 13C NMR spectrum of methyl propanoate, CH3CH2CO2CH3(figure). TMS CH-cH 2 1 120 200 180 160 140 100 20 40 O ppm 60 Chemical shift (8) Intensity
-
What is the difference between an optimistic approach and a pessimistic approach to decision making under assumed uncertainty?
-
American and European bricks-and-mortar retailing is increasingly becoming dominated by "hyper marts," enormous stores that sell groceries, household goods, hardware and other products under one...
-
A quality-control program at a plastic bottle production line involves inspecting finished bottles for flaws such as microscopic holes. The proportion of bottles that actually have such a flaw is...
-
X enters into an agreement with Y whereby X was to deliver some goods to Y. The agreement mentions that Y would provide X with a letter of credit by way of compensation, the amount of which would be...
-
Beasley Company makes three types of exercise machines. Data have been accumulated for four possible overhead drivers. Data for these four possible drivers are shown in rows 3 to 7 of the following...
-
Happy birthday! You are 30 years old today. You want to retire at age 60. You want to have $2,100,000 at retirement. Realistically, you know that the most that you can save from your 31st birthday...
-
Do you remember Brenda who used to be my boss? Stephanie queried. She once said that shed invest in my business if I was really going to do it, so it looks like I can get some equity investment. I...
-
Long-range coupling between protons more than two carbon atoms apart is sometimes observed when ? bonds intervene. An example is found in 1-methoxy-1-buten-3-yne. Not only does the acetylenic proton,...
-
The 1H and 13C NMR spectra of compound A, C8H9Br are shown. Propose a structure for A, and assign peaks in the spectra to your structure. TMS O ppm 10 8. 6. Chemical shift (8) TMS 200 180 160 140 120...
-
Explain why the purchase of supplies is not recorded in the special columns of the purchases journal, store equipment is not recorded in the journals Purchases Debit column, and the General Debit...
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
need step by step instruction about creating this: in NX12 PART NAME: BRACKET ALL FILLETS R .313 ALL ROUNDS R .625 2X .500 1/500 2.875 9.500 4750 2875 $500 3.000 750 GENTERED IN OBJECT 2.375
-
8. Convert the angle - 7t from radian measure into degree measure. Show some work. 4
-
4. Variance Analysis. (CPA, adapted) The H. G. Company uses a standard cost system in accounting for the cost of one of its products. < The Budget is based on normal capacity of monthly production of...
-
What is the difference between metric and nonmetric scales? Give an example of each.
-
Suppose a population of bacteria doubles every hour, but that 1.0 x 106 individuals are removed before reproduction to be converted into valuable biological by-products. Suppose the population begins...
-
A KNO 3 solution containing 45 g of KNO 3 per 100.0 g of water is cooled from 40 C to 0 C. What happens during cooling?
-
Predict the products of the following reactions. (a) allyl bromide + cyclohexyl magnesium bromide (b) cyclopentadiene + anhydrous HCI (c) 2-methylpropene + NBS, light (d) Furan + trans 1,...
-
Show how the reaction of an allylic halide with a Grignard reagent might be used to synthesize the following hydrocarbons. (a) 5-methylhex-1-ene (b) 2,5,5-trimethylhept-2-ene (c)...
-
Draw the important resonance contributors for the following cations, anions, and radicals. (a) (b) (c) (d) (e) (f) (g) (h) CH CH 2 - CH2 OF OCH3
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


