On reaction with Cl 2 in the presence of light, an unknown compound with the formula C
Question:
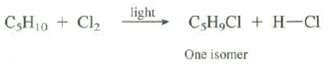
On reaction with Cl2 in the presence of light, an unknown compound with the formula C5H10 gives only one isomer of C5H9Cl (see problem 2.39). What is the DU of the unknown compound? Show the structure of the unknown compound and the product of its reaction with Cl2.
Transcribed Image Text:
CsH₁0 + Cl₂ light C,H,Cl + H-CI One isomer
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
The DU of C5H0 25 2 122 1 so the unknown has one rin...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
An unknown compound of molecular formula C5H9NO gives the IR and NMR spectra shown here. The broad NMR peak at δ7.55 disappears when the sample is shaken with D2O. Propose a structure,...
-
On heating 1,2,4-butanetriol in the presence of an acid catalyst, a cyclic ether of molecular formula C4H8O2 was obtained in 81-88% yield. Suggest a reasonable structure for this product.
-
In the presence of an acid catalyst, acetaldehyde forms a trimer known as paraldehyde. Because it induces sleep when it is administered to animals in large doses, paraldehyde is used as a sedative or...
-
Marcus is the HR manager for United Airlines, an Illinois-based company. One of his employees has recently become disabled and is unable to fulfill the essential functions of his current position,...
-
What are the advantages and disadvantages of basing individual incentives on company-wide performance?
-
Iqbal Corporation uses the lower of FIFO cost and net realizable value method on an individual item basis, applying the direct method. The inventory at December 31, 2016, included product AG....
-
Rental car call center study. A worldwide rental car company receives about 10,000 customer complaint calls per month at its European call center. In an effort to reduce the proportion of issues that...
-
Conventional wisdom says that one should measure a managers investment performance over an entire market cycle. What arguments support this convention? What arguments contradict it?
-
According to the 150% SDA model, what is the monthly default rate in month 22? Express your answer as a number rounded to eight decimal points (for example if your answer is 0.00511111%, write...
-
MC Liquidation Warehouse sells goods to retail stores and extends credit terms of 1/10, n/30. MC Liquidation has a stated policy of no returns, all sales are final. The company uses a perpetual...
-
One of the isomers of C 5 H 12 reacts with Cl 2 in the presence of light to produce three isomers of C 5 H 11 Cl: This reaction replaces am one of the hydrogen?s of C 5 H 12 with a Cl. What arc the...
-
Explain how the dipole moment for CH3Cl ( = 1.9 D) can be larger than the dipole moment for CH3F ( = 1.8 D).
-
Which of the choices would be most appropriate here? A. NO CHANGE B. I love how people seem so different and are so similar. C. People seem so different, so I love how they end up being so similar....
-
Encouraging you to sit back and watch a full hour of one of your favorite shows on prime-time television. However, instead of getting up during the commercial break or fast forwarding through the...
-
A family member has been recently diagnosed with a heart condition that requires replacing a heart valve. She points out that if she goes to India, the surgery cost is about 60% cheaper on average...
-
Based on the case of Bowers Machine Parts. Critically analyze why people were not doing their best and critically explain why hiring a consultant might solve the issue. Justify your answer by using...
-
Identify a few strategies for sustainability effectiveness. Should sustainability be a corporation's top priority? Why or why not? What are the challenges associated with implementing sustainable...
-
Answer the following questions for the topic you want to write about. Type your answers in a separate Word document. What is the issue or debatable idea you might write about? What is debatable about...
-
What, specifically, can a manager do to recognize employees for accomplishing a goal?
-
A random sample of 10 houses heated with natural gas in a particular area, is selected, and the amount of gas (in therms) used during the month of January is determined for each house. The resulting...
-
A force of 40 N is required to hold a spring that has been stretched from its natural length of 10 cm to a length of 15 cm. How much work is done in stretching the spring from 15 cm to 18 cm?
-
Rank the following substances in order of increasing acidity: (a) (CH3)2CHOH, HC CH, (CF3)2CHOH, CH4OH (b) Phenol, p-methyl phenol, p-(trifluoromethyl) phenol (c) Benzyl alcohol, phenol, p-hydroxy...
-
P-Nitro benzyl alcohol is more acidic than benzyl alcohol but p-methoxy benzyl alcohol is less acidic. Explain.
-
Predict the products of the following reactions: CH (a) 1. CHCH2- 2. NaOH, H2O2 "CH (b) 1. HglOAc)2. 0 2. NABH4 (c) CCH2CH2CH2 CH2CH2CH2CH3 C=C 1. Os04 2. NaHSO3, H20 -
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


