One of the isomers of C 5 H 12 reacts with Cl 2 in the presence of
Question:
One of the isomers of C5H12 reacts with Cl2 in the presence of light to produce three isomers of C5H11Cl:
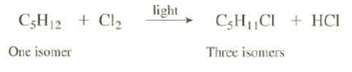
This reaction replaces am one of the hydrogen?s of C5H12 with a Cl. What arc the structures of the C5H12, isomer and the three C5H11Cl isomers produced from it?
Transcribed Image Text:
C5H12 + Cl₂ One isomer light CH₁CI+HCI Three isomers
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Draw all the isomers three of C 5 H 12 Then draw the poss...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
2,3-Dimethylbutane reacts with bromine in the presence of light to give a monobrominated product. Further reaction gives a good yield of a dibrominated product. Predict the structures of these...
-
Myo-lnositol, one of the isomers of 1, 2, 3, 4, 5, 6-hexahydroxvcyclohexane, acts as a growth factor in both animals and microorganisms. Draw the most stable chair conformation ofmyo-inositol. . " ....
-
Hydrogen gas is produced when zinc reacts with sulfuric acid: Zn(s) + H2SO4 (aq) ZnSO4 (aq) + H2(g) If 159 mL of wet H2 is collected over water at 24oC and a barometric pressure of 738 torr, how...
-
Arrange the following events in the correct temporal sequence during eukaryotic cell division, starting with the earliest: (a) condensation of the chromosomes, (b) Movement of chromosomes to the...
-
Explain how return on investment and residual income can be used as part of a responsibility accounting system.
-
A company's accounts receivable amounted to 150,000. Its receivable turnover is 10x and inventory is maintained at a level equal to 15 days' sales. The selling price per unit is P100.00 and has a...
-
Monitoring surgery complications. An article on the use of control charts for monitoring the proportion of post operative complications at a large hospital was published in the International Journal...
-
A new firm announces that it will invest $150 million in projects each year forever. All projects are expected to generate a 15 percent rate of return on its beginning-of-period book value each year...
-
Water Sport Inc. manufactures a small personal water tube used for children learning to swim. Management is now preparing detailed budgets for the third quarter, July through September, and has...
-
Auditors for the Internal Revenue Service (IRS) scrutinize income tax returns after they have been prescreened with the help of computer tests for normal ranges of deductions claimed by taxpayers....
-
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is...
-
On reaction with Cl 2 in the presence of light, an unknown compound with the formula C 5 H 10 gives only one isomer of C 5 H 9 Cl (see problem 2.39). What is the DU of the unknown compound? Show the...
-
What is the balanced scorecard? What perspectives are considered in select ing performance measures for the balanced scorecard? LO.1
-
What is the discount rate? PV = 7 0 0 ; t = 5 year period; FV = 1 0 0 0
-
How is planning illustrated in this case story? How is strategic management illustrated in this case story? The new CEO stated that the CEO's job is to give employees a point of view. Explain what...
-
Explain the Following Questions: 1. What essential characteristics exist in a proper understanding of "personal mastery," so that as an individual achieves greater progress in this discipline, they...
-
Few people want to eat discolored french fries. Potatoes are kept refrigerated before being cut for french fries to prevent spoiling and preserve flavor. But immediate processing of cold potatoes...
-
Part 3 of 4 Points: 0.49 of 1 Compute P(X) using the binomial probability formula. Then determine whether the normal distribution can be used to estimate this probability. If so, approximate P(X)...
-
What motivates you to accomplish difficult but important goals?
-
Draw two scatterplots, one for which r = 1 and a second for which r = 21.
-
Find the approximate area of the region bounded by the curves y = x/x 2 + 1 and y = x 4 x.
-
Give IUPAC name for the following compounds: (c) , (b) (a) CHCH,CHCHCH CH CH2CH2CH3 -CH CH C (e) . (f) (d) Br Br
-
Draw structures corresponding to the following IUPAC name: (a) (Z)-2-Ethyl-2-buten-1-o1 (b) 3-Cyclohexen-1-o1 (c) trans-3-Chlorocycloheptanol (d) 1, 4-Pentanediol (e) 2, 6-Dimethylphenol (f)...
-
The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point with increasing substitution of the OH-bearing carbon. How might you account for this trend?...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


