One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2.
Question:
One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (1) Protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+.
(a) Draw two resonance structures of diazomethane, and account for step 1.
(b) What kind of reaction occurs in step2?
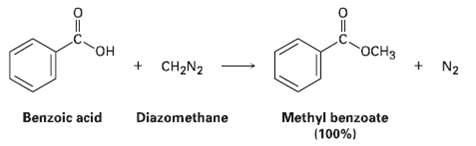
Transcribed Image Text:
OCH3 + N2 он + CH2N2 Methyl benzoate (100%) Benzoic acid Diazomethane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a HCNEN HCNN Resonance forms show that the carbon of diazo...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Diazomethane can be used to convert a carboxylic acid into a methyl ester. Propose a mechanism for this reaction. a carboxsyllc diazomethane RCOH + CH2N2 RCOCH3 N2 a methyl acid ester
-
Draw two resonance structures for diazomethane, CH2N2. Show formal charges. The skeletal structure of the molecule is C N N
-
A carboxylic acid has two oxygen atoms, each with two nonbonding pairs of electrons. (a) Draw the resonance forms of a carboxylic acid that is protonated on the hydroxyl oxygen atom. (b) Compare the...
-
The University of Professional Studies, Accra (UPSA) is a public university in Ghana. UPSA is the first university in Ghana to provide both academic and business professional education. The...
-
Are you a member of the "thumb generation"? Can you work the keyboard of your smartphone faster than most people can speak? The term thumb generation was coined in South Korea and Japan and is...
-
Explain the ways in which social class and income can serve as predictors of consumption.
-
20. How does a corporations decision to pay dividends affect its overall tax rate?
-
Childress Company produces three products, K1, S5, and G9. Each product uses the same type of direct material. K1 uses 4 pounds of the material, S5 uses 3 pounds of the material, and G9 uses 6 pounds...
-
The Juliuss Company is applying the Miller-Orr model to its cash management. They determined that the return point is $ 15,000, the lower limit is $7,000, and the upper limit is $26,000. Explain what...
-
In 2009, Jay Inc. and Vee Ltd., both Canadian manufacturing companies, decided to form a joint venture to build and operate a manufacturing plant in Asia. Their joint venture would have lower...
-
Tranexamic acid, a drug useful against blood clotting, Is prepared commercially from p-methyl Benzonitrile. Formulate the steps likely to be used in the synthesis. (Don?t worry about cis-trans...
-
The hydrolysis of a biological thioester to the corresponding carboxylate is often more complex than the overall result might suggest. The conversion of succinyl CoA to succinate in the citric add...
-
What is lateral cycling and why is it important to marketing?
-
Prepare the entries to record the transaction 2 A company has three employees, each of whom has been employed since January 1 earns $2750 per month and is paid on the last day of each month On March...
-
Pet Emporium had a robbery on the weekend in which a large amount of inventory was taken. The loss is covered completely by insurance. A physical inventory count determined that the cost of the...
-
In a test taken by a class of 50 students, the average was 1500 with a standard deviation of 40. What 2 scores capture the middle 60% of the students?
-
For questions 1-8, let P = (-2, 5) and Q = (4,8). 1. Find the distance from the point P to the point Q. 2. Find the midpoint of the line segment joining P and Q. 3. Find the slope of the line PQ. 4....
-
True/False Indicate whether the statement is true or false. ____ 1. In accounting, to value means to record a transaction or event. ____ 2. The recognition issue deals with when a business...
-
How does the resource-based view of the firm provide a superior means of evaluating a companys competitive advantage? AppendixLO1
-
Refer to Example 9.15. Add the following functionality to this program: Allow the user to enter the cost of a gallon of gas on each trip and use a function, Cost() to calculate the cost of purchasing...
-
Write a short paragraph describing chemical bonding according to the Lewis model, valence bond theory, and MO theory. Indicate how the theories differ in their description of a chemical bond and...
-
Explain each of the following observations. The allene 2, 3-heptadiene can be resolved into enantionmers, but the cumulene 2, 3, 4-heptatriene cannot.
-
Using the Huckel 4n + 2 rule, determine whether each of the following compounds is likely to be aromatic. Explain how you arrived at the -electron count in each case. (a) (b)
-
The following compound is not aromatic even though it has 4n + 2 electrons in a continuous cyclic array. Explain why this compound is not aromatic.
-
The tolal landed coet with the order gaantly sire of 6,000 unts is 4 (Enter your response roundod to the nearest dolar)
-
Boyne Inc. had beginning inventory of $12,000 at cost and $20,000 at retail. Net purchases were $120,000 at cost and $170,000 at retail. Net markups were $10,000, net markdowns were $7,000, and sales...
-
Apple inc. CONDENSED CONSOLIDATED BALANCE SHEETS (Unaudited) (In milions, except number of shares which are reflected in thousands and par value) LABILITES AND SHAREHOLDERS' EQUITY: Current...

Study smarter with the SolutionInn App


