Outline a synthesis of each of the following compounds from the indicated starting material. (a) CH3CH2CH2CH2CH2CH2D from
Question:
Outline a synthesis of each of the following compounds from the indicated starting material.
(a) CH3CH2CH2CH2CH2CH2D from l-hexanol
(b)
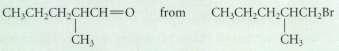
Transcribed Image Text:
CHCH,CH CHCH-O from CH,CH,CH,CHCH2Br CH3 CHy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a The deuteriumcontaining alkane can be prepared by p...View the full answer

Answered By

Jacob Festus
I am a professional Statistician and Project Research writer. I am looking forward to getting mostly statistical work including data management that is analysis, data entry using all the statistical software’s such as R Gui, R Studio, SPSS, STATA, and excel. I also have excellent knowledge of research and essay writing. I have previously worked in other Freelancing sites such as Uvocorp, Essay shark, Bluecorp and finally, decided to join the solution inn team to continue with my explicit work of helping dear clients and students achieve their Academic dreams. I deliver, quality and exceptional projects on time and capable of working under high pressure.
4.90+
1263+ Reviews
2858+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which ordering gives the correct sequence of values of the standard enthalpies of atomization of the elements. (A) B < Al < TI (B) Tl < Ga < B (C) In > Al > B (D) Ga < Al < B
-
Outline a synthesis of each of the following compounds from isobutyric acid (2-methylpropanoic acid) and any other necessary reagents. (a) (b) 0 CH) CHC OCH, CCHCH isobutyrophenone
-
Starting with styrene, outline a synthesis of each of the following: (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) (k) (l) (m) (n) Cl Cl CHs OH OH C&Hs OH OH C6H5 Br C6H5 caHs CeHs C6H5
-
XYZ Co. It is currently trading at $5 per share and has announced a $0.50 per share dividend payable next year. Using historical information, one analyst estimates XYZ Co.'s dividend growth rate is...
-
Select a product suppose that the government places a mandated price ABOVE the equilibrium price, based on your research how would this affect the market equilibrium, explain your view.
-
The Allen Oil Company incurred the following costs and had the other transactions shown below for the years 2005 and 2006. The company uses the successful efforts method of accounting. 2005 a. Paid...
-
Scope ______ is often achieved by a customer inspection and then sign-off on key deliverables. a. acceptance b. validation c. completion d. close-out LO.1
-
Mike Polanski is 30 years of age and his salary next year will be $40,000. Mike forecasts that his salary will increase at a steady rate of 5% per annum until his retirement at age 60. a. If the...
-
any answer will get a like! Schedule of Cash Collections of Accounts Receivable Furry Friends Supplies Inc, a pet wholesale supplier, was organized on May 1. Projected sales for each of the first...
-
On January 1, 2020, Xiamen Company made amendments to its defined benefit pension plan that resulted in 62,800 yuan of past service cost. The plan has 5,130 active employees with an average expected...
-
In each of the following cases, imagine that the two reactants shown are allowed to reirct,in the presence of alcohol dehydrogenase. Tell whether the ethanol formed is chiral. If the ethanol is...
-
Show how one can work backwards from the target step-by-step to the "present situation" in each of the following real-life problems. Target: Successfully financing a college education. Present...
-
Montgomery Ward operates a retail department store chain. It filed for bankruptcy during the first quarter of Year 12. Exhibit 3.27 presents a statement of cash flows for Montgomery Ward for Year 7...
-
Use z scores to compare the given values. Based on sample data, newborn males have weights with a mean of 3240.3 g and a standard deviation of 675.8 g. Newborn females have weights with a mean of...
-
Compare the given pulse rates of the females and males using boxplots. Female 80 94 Male 84 74 50
-
In Exercises 29-32, compute the mean of the data summarized in the frequency distribution. Also, compare the computed means to the actual means obtained by using the original list of data values,...
-
Floyd's Bumpers has distribution centers in Lafayette, Indiana; Charlotte, North Carolina; Los Angeles, California; Dallas, Texas; and Pittsburgh, Pennsylvania. Each distribution center carries all...
-
In this assignment you are asked to open the Excel Spreadsheet for YP Enterprises (March 2019) and complete the section entitled Ratios 2019 (highlighted in yellow). This will require you to...
-
Do the interest rates on Treasury securities include a DRP? An LP? A PRP? Explain your answer.
-
Provide an example of an aggressive accounting practice. Why is this practice aggressive?
-
Monoamine oxidase (MAO) is an enzyme that catalyzes the oxidation of certain biologically important amines. One form of the enzyme catalyzes the following oxidation of serotonin, an important...
-
When the hydration of fumarate is catalyzed by the enzyme fumarase in D 2 O, only (2S,3R)-3-deuteriomalate is formed as the product. (EachCO 2 group is the conjugate base of a carboxylic acid group.)...
-
In a laboratory are found two different compounds: A (melting point 24.7C) and B (melting point 21C). Both compounds have the same molecular formula (C 7 H 14 O), and both can be resolved into...
-
X Your answer is incorrect. Flounder Consulting Corp. company records revealed the following for the current year: What was the net cash flow from operating activities for the year? $ 0 $ 9 8 0 0...
-
Assume that interest rate parity holds. The U.S. fiveyear interest rate is 0.08 annualized, and the Mexican fiveyear interest rate is 0.05 annualized. Todays spot rate of the Mexican peso is $0.21....
-
find the NSP of a whole life insurance.6 with $100,000 Death benefits, for a female aged 105 years, if i=10%? (use Australian life Tables 2005-07) find the NSP of a whole life insurance.6 with...

Study smarter with the SolutionInn App


