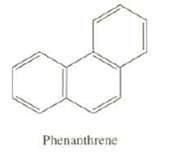
Phenanthrene has five total resonance structures. One is shown here. Draw the other four. Which carbon-carbon bond
Question:
Phenanthrene has five total resonance structures. One is shown here. Draw the other four. Which carbon-carbon bond of Phenanthrene would your predict to be the shortest?
Transcribed Image Text:
Phenanthrene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
The five resonance structures are quite similar and are expected to m...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The resonance structures of carbon monoxide are shown below. Show how each structure can be converted into the other using the curved-arrow notation. :C-: :C=0:
-
Draw four reasonable resonance structures for the PO3F2- ion. The central P atom is bonded to the three O atoms and to the F atom. Show formal charges.
-
Draw contributing resonance structures for each of the following species, and rank the structures in order of decreasing contribution to the hybrid: a. b. c. d. e. f. CH3C-CH CHCH3 CH3 0 CH3COCH3 +OH...
-
You are the assistant vice president in charge of production for a firm that produces computers. Your firm's production function is f(L,K) = min (L,K) Where L and K are the quantities of the two...
-
Explain why it is important that the activities used to develop a flexible overhead budget are accurate cost drivers?
-
1. Jordon James started JJJ Consulting on January 1. The following are the account balances at the end of the first month of business, before adjusting entries were recorded. Accounts...
-
Explain what makes an effective marketing plan.
-
Suppose that in past years the average price per square foot for warehouses in the United States has been $32.28. A national real estate investor wants to determine whether that figure has changed...
-
Net sales for the month are $800,000 and bad debts are expected to be 1.5% of net sales. The company uses the percentage of sales method in estimated bad debt expense. If the Allowance for Doubtful...
-
The position of a particle as a function of time is given by r(vector) = (5.0i + 4.0j)t 2 m, where t is in seconds. a. What is the particles distance from the origin at t = 0, 2, and 5 s? b. Find an...
-
Draw resonance structures for this anion. Remember, sulfur can have 10 or even 12 electrons in its valence shell. :0: 24 CH-S2+0 :0:
-
Show the three additional resonance structures for anthracene. Discuss whether the experimental bond lengths shown in the following structure are in accord with predictions based on these resonance...
-
In problem use the Adams-Bashforth-Moulton method to approximate y(1.0), where y(x) is the solution of the given initial-value problem. First use h = 0.2 and then use h = 0.1. Use the RK4 method to...
-
Which one of the following is not a part of the Deployment phase of a machine learning development project? Explain what phase(s) address this issue, and why then? Training end users to incorporate...
-
Assist with the following discussion: Topic Discussion #1B: The first half of the term is devoted to leaders preparing themselves for leadership. Peter Senge and his coauthors discuss in The Dawn...
-
You are managing an employee who is not a self-starter, and thus you need to devise a plan to effectively lead this employee. Draft a one page (Times New Roman 12) single space response (plus title...
-
Ontario's minister of training, colleges and universities defended changes to post-secondary education on Monday, saying recently announced decisions are all about the making the system more...
-
"The power of globalization is not about leveraging economies of scale. It's about leveraging economies of knowledge and coordination figuring out how not to reinvent the wheel everywhere you do...
-
1 What needs do they seem to be aiming to meet? Would they meet your needs? Visit the websites of companies that interest you, perhaps as possible places to work. www.childbase.co.uk...
-
14. In testing the existence assertion, an auditor ordinarily works from the a. Financial statements to the accounting records. b. General journal to the general ledger. c. Supporting evidence to the...
-
Determine whether the series is convergent or divergent. 8 n=1 cos n n cos 1 1 + cos 2 2 + cos 3 3 +
-
Draw the five cycloalkanes with the formula C5H10.
-
Draw two constitutional isomers of cis-1, 2-dibromo-cyclopentane.
-
Draw a stereoisomer of trans-1, 3-climethylcyclobutane.
-
This short exercise demonstrates the similarity and the difference between two ways to acquire plant assets. (Click the icon to view the cases.) Compare the balances in all the accounts after making...
-
Balance sheet and income statement data for two affiliated companies for the current year appear below: BALANCE SHEET As at December 31, Year 6 Albeniz Bach Cash $ 40,000 $ 21,000 Receivables 92,000...
-
please reference excel cells Caroll Manufacturing company manufactures a single product. During the past three weeks, Caroll's cost accountant observed that output costs varied considerably. The...

Study smarter with the SolutionInn App


