Question: Polarizability of atomic hydrogen consider a semi classical model of the ground state of the hydrogen atom in an electric field normal to the plane
Polarizability of atomic hydrogen consider a semi classical model of the ground state of the hydrogen atom in an electric field normal to the plane of the orbit (Fig. 25), and show that for this model a = a3H;, where aH, is the radius of the unperturbed orbit. Note: If the applied field is in the x direction, then the x component of the field of the nucleus at the displaced position of the electron orbit must be equal to the applied field. The correct quantum-mechanical result is larger than this by the factor 9/2. (We are speaking of a0 in the expansion a = a0, + a1 E + . . . .) We assume x H. One can also calculate al on this model.
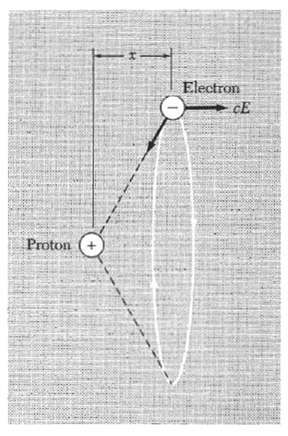
Proton Electron CE
Step by Step Solution
3.19 Rating (171 Votes )
There are 3 Steps involved in it
cul e ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
14-P-S-S-G-P (112).docx
120 KBs Word File


