Predict the aldol reaction product of the followingcompounds: (c) (b) (a) CCH-CH2CH CH3
Question:
Predict the aldol reaction product of the followingcompounds:
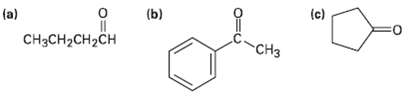
Transcribed Image Text:
(c) (b) (a) CнзCH-CH2CH CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (23 reviews)
1 Form the enolate of one molecule of the carbonyl compound CH3CHCHICH OH CH3CHCHCH 2 ...View the full answer

Answered By

Dinesh F
I have over 3 years of professional experience as an assignment tutor, and 1 year as a tutor trainee.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the products from each of the following aldol reactions. (a) (b) (c) (d) (e) H NaOH H20 H H NaOH H2O o'H H HOEN NaOH H2O H NaOH H2O
-
Predict the products of the following aldol condensations. Show the products both before and after dehydration. (a) (b) (c) (d) (e) (f) CH3 TOH CH CH2-C-H CH TOH Ph-C-CH+ OH
-
The following reaction does not produce the product shown. (a) Predict the major product from the conditions shown above, and write a detailed mechanism for its formation. (b) What reaction...
-
Neer Department Store uses the retail inventory method to estimate its monthly ending inventories. The following information is available for two of its departments at August 31, 2011. Sporting Goods...
-
Your client is the planning office of a major university. Part of the job of the planning office is to forecast the annual donations of alumni through the university's long-established giving...
-
Consider the drug patch shown in Problem 26.4. The patch consists of a pure solid drug source mounted on top of a water-swollen polymer, which acts as a controlled diffusion barrier. The square patch...
-
Explain the accounting entries for the formation of a partnership. LO5
-
On April 15, 2015, Sampson Consulting provides services to a customer for $ 110,000. To pay for the services, the customer signs a three- year, 12% note. The face amount is due at the end of the...
-
Instructions: Please complete the 2020 federal income tax return for Sarah Hamblin. Be sure to include only required tax forms when completing the tax return. Due date: Wednesday October 20, 2021...
-
The accountant for Davidson, Inc. prepared the following analysis of its inventory at year end: Item Units Cost per Unit Net Realizable Value RSK-89013 520 $38 $44 LKW-91247 329 49 45 QEC-57429 462...
-
Carboxylic acids (RCO 2 H; p K a 5) are approximately 10 11 times more acidic than alcohols (ROH; pK a 16). In other words, a carboxylate ion (RCO 2 ) is more stable than an alkoxide ion (RO )....
-
Using curved arrows to indicate the election flow in each step, show how the base-catalyzed reverse aldol reaction of 4-hydroxy-4-methly-2-pentanone takes place to yield 2 equivalents of acetone.
-
The following transactions apply to Gupta Co. for 2010, its first year of operations. 1. Received $50,000 cash in exchange for issuance of common stock. 2. Secured a $100,000, 10-year installment...
-
1. create a concept map for 0D, 1D, 2D and 3D crystals 2. write down the formulas for quantifying numbers of defects
-
\fNOTES TO CONSOLIDATED FINANCIAL STATEMENTS OF AMERICAN AIRLINES GROUP INC . Commitments , Contingencies and Guarantees ( 2 ) Aircraft and Engine Purchase Commitment Under all of our aircraft and...
-
Critical Values. In Exercises 41-44, find the indicated critical value. Round results to two decimal places. 41. Z0.25 42. Z0.90 43. Z0.02 44. 20.05
-
Use the following information for questions 1 and 2. Caterpillar Financial Services Corp. (a subsidiary of Caterpillar) and Sterling Construction sign a lease agreement dated January 1, 2020, that...
-
In todays social and business environments, some organizations only talk the talk regarding ethics and ethical conduct rather than walk the ethical organizational path. In what ways can ethical and...
-
Differences in background is a common obstacle to effective communication because of all of the following except: A. Individuals may not share the same education levels. B. Individuals may not share...
-
Smiths Family Fashions implemented a balanced scorecard performance measurement system several years ago. Smiths is a locally owned clothing retailer with fashions for men, women, teens, and...
-
Draw the Lewis structure for HCSNH 2 . (The carbon and nitrogen atoms are bonded together, and the sulfur atom is bonded to the carbon atom.) Label each bond in the molecule as polar or nonpolar.
-
Calculate the specific rotations of the following samples taken at 25 C using the sodium D line. (a) 1.00 g of sample is dissolved in 20.0 mL of ethanol. Then 5.00 mL of this solution is placed in a...
-
Tartaric acid has a specific rotation of +12.0o. Calculate the specific rotation of a mixture of 68% (+)-tartaric acid and 32% (-) tartaric acid.
-
The specific rotation of (S)-2-iodobutane is +15.90o. (a) Draw the structure of (S)-2-iodobutane. (b) Predict the specific rotation of (R)-2-iodobutane. (c) Determine the percentage composition of a...
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


