Predict the complete NMR spectrum of 1, 2-dichloropropane under each of the following assumptions. Note that protons
Question:
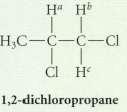
Predict the complete NMR spectrum of 1, 2-dichloropropane under each of the following assumptions. Note that protons and Hb and Hc are diastereotopic.
Jab = Jac)
Transcribed Image Text:
Ha Hb HC-C-C-Cl ClHc 1,2-dichloropropane
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 38% (13 reviews)
Because protons H b and H c are diastereotopic they are chemically nonequivalent therefore countin...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The 200-MHz 1H NMR spectrum of 1, 4-dimethylbenzene looks exactly like that of CH3OCH2CN except the chemical shifts of the two peaks are 2.2 ppm and 7.0 ppm. Assign the peaks to the appropriate...
-
How many peaks would you expect in the 1H NMR spectrum of 1, 4-dimethyl-benzene para-xy1ene or p-xylene)? What ratio of peak areas would you expect on integration of the spectrum? Refer to Table 13.3...
-
What changes would you expect in the 13C NMR spectrum of 1-bromopropane upon cooling the compound to very low temperature?
-
Tim plays a computer game. Each game is a win or a loss. He wins three fifths of his first 40 games. He then wins his next 12 games. For all 52 games, Required: work out the ratio wins: losses Give...
-
Saul Cervantes has just purchased some equipment for his landscaping business. He plans to pay the following amounts at the end of the next five years: $10,617, $8,364, $12,849, $14,273, and $8,517....
-
Show that you must earn a 25 percent return to offset a 20 percent loss.
-
What are the accounting ramifications of each of the two following situations involving the payment of contingent consideration in a purchase? a. P Company issued 100,000 shares of its $50 fair value...
-
Foxx Companys cost structure is dominated by variable costs with a contribution margin ratio of .25 and fixed costs of $100,000. Every dollar of sales contributes 25 cents toward fixed costs and...
-
Net Devices Inc. The following balance sheets and income statements are for Net Devices Inc., a manufacturer of small electronic devices, including calculators, personal digital assistants and mp3...
-
Dry Supply is a wholesaler of dry cleaning equipment, cleaning supplies, and laundry soap. This company is located in Kansas, it has been in business for over 50 years. Anne Schippel, is a business...
-
What is the reduction in shielding of Protons b relative to the protons of TMS in magnetic field units of gauss? The applied field in a 300 MHz NMR spectrometer is 70,500 gauss.
-
Identify the following two isomeric alkyl halides (C5H11Br) from their 300-MHz NMR spectra, which are as follows: Compound A: 0.91 (6/7, d, J = 6 Hz); 1.7-1.8 (3H, complex); 3.42 (2H, t, J = 6 Hz)...
-
a. What is a financial option? What is the single most important characteristic of an option? b. Options have a unique set of terminology. Define the following terms: (1) Call option; (2) Put option;...
-
1. What does the phrase "cost of quality" mean? How might using this statement assist a company in addressing its quality issues? 2. What key distinctions exist between total quality human resource...
-
Does productivity in terms of output per labor our insure a company will be profitable? Why or why not? What questions should be asked to test whether productivity has increased? How do these answers...
-
How do the four Ps of marketing (product, price, promotion, place) differ in international markets?
-
Do you agree with the societal or political forces? Why or why not? Support your assertions with credible sources
-
How do the global transformational leadership models comprise a work environment that sees the need for change and embraces the new changes?Explain
-
Is the CCC affected by short-term financing plans? Explain your answer.
-
An investor sells a European call on a share for $4. The stock price is $47 and the strike price is $50. Under what circumstances does the investor make a profit? Under what circumstances will the...
-
Use resonance arguments to explain why (a) P-methoxybenzaldehyde is more basic than p-nitrobenzaldehyde. (b) 3-buten-2-one is more basic than 2-butanone.
-
(a) Write a curved-arrow mechanism for the hydroxide-catalyzed hydration of acetaldehyde. (b) Write a curved-arrow mechanism for the decomposition of acetone cyanohydrin (Eq. 19.15) in aqueous...
-
(a) Write an S N 1 mechanism for the solvolysis of CH 3 OCH 2 Cl [(chloromethoxy)methane] in ethanol; draw appropriate resonance structures for the carbocation intermediate. (b) Explain why the alkyl...
-
You plan to buy a house for $325,000 today. If the house is expected to appreciate in value 8% each year, what will its value be seven years from now?
-
A designated beneficiary of an ABLE account must be ___________ in order to meet the special rules that apply to the increased contribution limit authorized under the Tax Cuts and Jobs Act? a. an...
-
Stans wholesale buys canned tomatoes from canneries and sells them to retail markets Stan uses the perpetual inventory

Study smarter with the SolutionInn App


