Predict the major product(s) of the followingreactions: (b) (a) CH3CH2COCI AICI3 CH3CH2CI AICI3 (c) (d) N(CH2CH3}2 CO2H
Question:
Predict the major product(s) of the followingreactions:
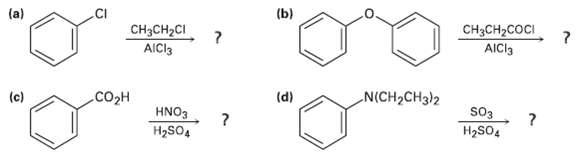
Transcribed Image Text:
(b) (a) CH3CH2COCI AICI3 CH3CH2CI AICI3 (c) (d) N(CH2CH3}2 CO2H HNO3 so3 H2SO4 H2SO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
b c CH3CHCl AICI 3 COH ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Predict the major product (or products) that would be obtained when each of the following compounds is nitrated: (a) (b) (c) OH CF CN SO3H OCH3 NO2
-
Predict the major product for each proposed Diels-Alder reaction. Include stereochemistry where appropriate. (a) (b) (c) Ph 0 Ph
-
Predict the major product formed when 2-bromobutane is subjected to dehydrobromination using sodium ethoxide in ethanol at 55oC.
-
Review TP1. Review current season ticket prices for one Major League Baseball team. Choose one season ticket price area to review. A. Determine what is recognized as per ticket revenue after each...
-
During 2016, John was the chief executive officer and a shareholder of Maze, Inc. He owned 60% of the outstanding stock of Maze. In 2013, John and Maze, as co-borrowers, obtained a $100,000 loan from...
-
What is revenue?
-
How are trading securities reported (valued) on the balance sheet? AppendixLO1
-
At July 31, Martinez Company has the following bank information: cash balance per bank $7,420, outstanding checks $762, deposits in transit $1,120, and a bank service charge $20. Determine the...
-
QUESTION FOUR: (15 marks) Kody Corporation uses a job-order costing system with a plant wide overhead rate based on machine hours. At the beginning of the year, the company made the following...
-
For hierarchical routing with 4800 routers, what region and cluster sizes should be chosen to minimize the size of the routing table for a three-layer hierarchy? A good starting place is the...
-
What product(s) would you expect to obtain from the followingreactions? (b) r. 1. HNO3, H2SO4 2. Fe, H30* "C Br H2/Pd NO2 (d) CI. (c) CH3CH2CH2CI MO 2 AICI3
-
Aromatic iodination can be carried out with a number of reagents, including iodine mono chloride, ICI. What is the direction of polarization of ICI? Propose a mechanism for the iodination of an...
-
The integrals we have seen so far suggest that there are preferred orders of integration for cylindrical coordinates, but other orders usually work well and are occasionally easier to evaluate....
-
You must select an orifice meter for measuring the flow rate of an organic liquid ( $\mathrm{SG}=0.8$, $\mu=15 \mathrm{cP}$ ) in a $4 \mathrm{in}$. sch 40 pipe. The maximum flow rate anticipated is...
-
A team of designers was given the task of reducing the defect rate in the manufacture of a certain printed circuit board. The team decided to reconfigure the cooling system. A total of 973 boards...
-
Level 98%: x1 = 49, n1 = 74, x2 = 62, n2 = 153 In Exercises 712, construct the confidence interval for the difference p1 p2 for the given level and values of x1, n1, x2, and n2.
-
Let X be a continuous random variable with the following PDF Find the MGF of X, M X (s). fx(x) = +) == 12e-1|2|1 e-A/).
-
The number of hours spent studying per day by a sample of 28 students In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 2 8 7 2 261 82 35 37 25 20 73 83...
-
What are the biggest challenges with exporting IWTs products?
-
Consider a closed, rigid tank with a volume of 0.8L, filled with cold water initially at 27C. The tank is filled such that there are no voids (air pockets) within. The initial pressure within the...
-
Two separate experiments are performed on a gas enclosed in a piston-and-cylinder device, both starting from the same initial state. The result of the first experiment is to be used to predict the...
-
Show all the steps in the mechanisms for these reactions. Don't forgot to use curved arrows to show the movement of electrons in eachstep. CH3 CH3 ) . + CHH ., + Br CH; CH3 H,SO, CH;CH,OCH,CH; + H;O...
-
Explain how both enantiomers of the product are formed in the reaction shown in problem 10.42c.
-
Show all the steps in the mechanism for thisreaction: CH,Br CH2OET ELOH OEt
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


