What product(s) would you expect to obtain from the followingreactions? (b) r. 1. HNO3, H2SO4 2. Fe,
Question:
What product(s) would you expect to obtain from the followingreactions?
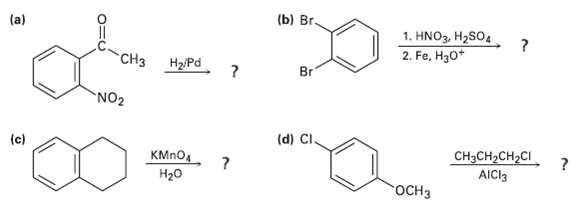
Transcribed Image Text:
(b) Вrг. 1. HNO3, H2SO4 2. Fe, H30* "CНз Br H2/Pd NO2 (d) CI. (c) CH3CH2CH2CI КMпO Н2о AICI3 гоСНз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
3 b Br CH3 NO oEthylaniline Catalytic hydrogenation reduces bo...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What stereoisomers would you expect to obtain from each of the following reactions? a. b. c. d. e. f. CH,CH CHs Br2 CH2C2 CH3 CH2CH3 CHCH CH Pt/c H3C CH2CH3 Br2 CH2Cl2 CH3 CH CH2C2 CH CH2CH H2 Pt/C...
-
What products would you expect to obtain from each of the following crossed Claisen condensations? (a) (b) Ethyl propanoate+ (1) NaOEt (2) H,o yl oxalate (1) NaOEt Ethyl acetate ethyl formate (2) H,O
-
What products would you expect to obtain from the complete hydrolysis of NAD? See page 547 for its structure.
-
As a bakery business continues to grow, cash flow has become more of a concern. The board of directors would like to maintain the market share price, so a discussion ensues about issuing a stock...
-
Mary Marble took out a homeowner's insurance policy on her residence. One of the policy provisions listed the insured's duties with respect to a loss. This provision required the policyholder to give...
-
In recent years, there have been many charges, some of them substantiated, that large companies have manipulated business transactions and accounting records to move income from one year to another...
-
How are short-term held-to-maturity securities reported (valued) on the balance sheet? AppendixLO1
-
Teri West operates her own catering service. Summary financial data for July are presented in equation form as follows. Each line designated by a number indicates the effect of a transaction on the...
-
The accounts listed below appeared in the December 31, 2020 trial balance of B&N Company Debit Credit Equipment KD 192,000 Accumulated Depreciation Equipment KD 60,000 Notes Payable 90,000 Prepaid...
-
1. What barriers to entry has Goodyear created or taken advantage of? 2. Goodyear has production facilities throughout the world. What competitive advantages might global production provide Goodyear?...
-
Rank the following aromatic compounds in the expected order of their reactivity toward FriedelCrafts alkylation. Which compounds are un-reactive? (a) Bromobenzene (b) Toluene (c) Phenol (d) Aniline...
-
Predict the major product(s) of the followingreactions: (b) (a) CH3CH2COCI AICI3 CH3CH2CI AICI3 (c) (d) N(CH2CH3}2 CO2H HNO3 so3 H2SO4 H2SO,
-
Identify the graph of each equation without completing the squares. 6x 2 + 3y 2 12x + 6y = 0
-
Write a program that solves either a) the Towers of Hanoi problem with up to 1000 disks, or, b) the Traveling Salesman problem with up to 10 cities. You may need to wait until you have read about...
-
Consider the E-R diagram in Figure 8-15b. a. What would be the identifier for the CERTIFICATE associative entity if Certificate Number were not included? b. Now assume that the same employee may take...
-
z = 1.1 for H a : < 149.6 Find the P-value that corresponds to the standard z-score, and determine whether the alternative hypothesis is supported at the 0.05 significance level.
-
An object is placed \(150 \mathrm{~mm}\) away from a converging thin lens that has a focal length of \(400 \mathrm{~mm}\). What are (a) the image distance and \((b)\) the magnification? (c) Draw a...
-
Let $M$ be the four-dimensional Minkowski space, with coordinates $x^{0}, x^{1}, x^{2}$, and $x^{3}$. Let us define a linear operator $*: \Omega^{r}(M) ightarrow$ $\Omega^{4-r}(M)$, such that...
-
What other applications are there for this wastewater technology?
-
d) For die casting processes: 1. What are the most common metals processed using die casting and discuss why other metals are not commonly die casted? 2. Which die casting machines usually have a...
-
Redo Problem 5.2 using Aspen Plus. Problem 5.2 t is desired to improve the thermal efficiency of the Rankine power generation cycle. Two possibilities have been suggested. One is to increase the...
-
Show how this synthesis might beaccomplished: Br CH3 CH3 HO from "CN -
-
What is wrong with these reactions explain. CI + NaOCH3 OCH3 + NaCl a) + HBr Br . b) CH,OH + CH;0 OCH3 CI d)
-
What is wrong with these synthesesexplain. 1) NaNH2, NH, (1) C=CCH3 a) CH3C=CH 2) -Br CH;CH,-NH, I b) CH;CH,I + NH3 CI + Br OCH, CH, + CH,O d) . .. Br + HBr e) CH3 CH3 H,SO, f)
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...

Study smarter with the SolutionInn App


