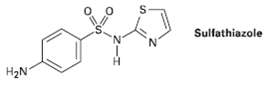
Propose a synthesis of the drug sulfathiazole from benzene and any necessaryamine. Sulfathiazole H2N
Question:
Propose a synthesis of the drug sulfathiazole from benzene and any necessaryamine.
Transcribed Image Text:
Sulfathiazole H2N
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
This reaction sequence is similar to the sequence used to synthesize sulfanilamide Key s...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a synthesis of 4, 4-dimethyl-2-pentyne (the compound in Problem 14.19) from an alkyl halide and an alkyne.
-
Propose a synthesis of the anti-inflammatory drug Fenclorac fromphenyl-cyclohexane. CI Fenclorac CHCO2H
-
Propose a synthesis of (R)-CH3CHN3CH2CH3, starting from (R)-2-chlorobutane.
-
Match the following ratios with the appropriate formula. Ratio or Rate Formula a. Income from operations Interest expense Acid-test Total liabilities Stockholders' equity Current b. Net income-...
-
Do you agree with how Adam Silver handled the situation? Do leaders really set the tone of an organization's values?
-
1. What were the possible risks of Louis Vuittons first-ever television advertising campaign? 2. In fall 2011, the euro/dollar exchange rate was ¬1 = $1.35. By spring 2015, the dollar had...
-
Did the restaurant act negligently in the serving of the hot chocolate?
-
A firm must decide between constructing a new facility or renting a comparable office space. There are two random outcomes for acquiring space, as shown in Figure PI 2-25. Each would accommodate the...
-
Salmone Company reported the following purchases and sales of its only product. Salmone uses a periodic inventory system. Determine the cost assigned to ending inventory using LIFO. Date Activities...
-
Assume that you estimate the Carlton's will need to pay $218,200 (Year 1 = $49,000, Year 2 = $52,500, Year 3 = $56,300, Year 4 = $60,400)for four years of tuition and room & board while Matthew...
-
What products would you expect from Hofmann elimination of the following amines? If more than one product is formed, indicate which ismajor. NH2 (b) NH2 (a) CH3CH2CH2CHCH2CH2CH2CH3 NHCH2CH3 (d) NH2...
-
Propose syntheses of the following compounds from benzene: (a) N, N-Dimethylaniline (b) p-Chloroaniline (c) m-Chloroaniline (d) 2, 4-Dimethylaniline
-
1. (1) When parents become old and destitute, the obligation of caring for them should be imposed on their children. (2) Clearly, children owe a debt to their parents. (3) Their parents brought them...
-
Consider the situation you addressed in Problem and Exercise 3. Create numeric cost estimates for each of the costs you listed. Calculate the net present value and return on investment. Include a...
-
The output power \(\dot{W}\) of a spinning shaft is a function of torque \(T\) and angular velocity \(\omega\). Use dimensional analysis to express the relationship between \(\dot{W}, T\), and...
-
In groups of three, pick a local healthcare organization with which you are familiar. Conduct a SWOT analysis on the organization. After completing the SWOT analysis, use the template in exhibit 8.12...
-
The Dean Door Corporation (DDC) manufactures steel and aluminum exterior doors for commercial and residential applications. DDC landed a major contract as a supplier to Walker Homes, a builder of...
-
In Exercises 47 and 48, write a two-column proof. GIVENmWYZ = m/TWZ = 45 PROVE SWZ = ZXYW SW X Y N T
-
Visit the Web sites of both the National Restaurant Associations Educational Foundation (www.nraef.org) and the Educational Institute of the American Hotel & Lodging Association (www.ei-ahla.org) and...
-
Reconsider Prob. 1474. In order to drain the tank faster, a pump is installed near the tank exit as in Fig. P1475. Determine how much pump power input is necessary to establish an average water...
-
At 70 K, CCl 4 decomposes to carbon and chlorine. The K p for the decomposition is 0.76. Find the starting pressure of CCl 4 at this temperature that will produce a total pressure of 1.0 atm at...
-
Divide the following compounds into groups that might be expected to exhibit similar chemical behavior: a. C4H10 b. CH3OCH3 c. C3H7OH d. C8H18 e. HOCH2CH2CH2OH f. CH3NH2 g. CH3CH2CH3 h. CH3OH i....
-
Write an equation similar to eq. 1.2 for the formation of a fluorine molecule from two fluorine atoms.
-
Using Table 1.6, write a structural formula for each of the following: a. An alcohol, C3H8O b. An ether, C4H10O c. An aldehyde, C3H6O d. A ketone, C3H6O e. A carboxylic acid, C3H6O2 f. An ester,...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


