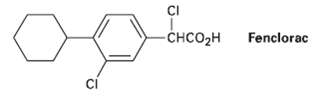
Propose a synthesis of the anti-inflammatory drug Fenclorac fromphenyl-cyclohexane. CI Fenclorac CHCO2H
Question:
Propose a synthesis of the anti-inflammatory drug Fenclorac fromphenyl-cyclohexane.
Transcribed Image Text:
CI Fenclorac CHCO2H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
PCC CHOH CHO CHCOH Br2 FeBr 3 Fenclorac 1 CH2O 2 H3O Cl F...View the full answer

Answered By

Yomna omer
I started my own tutoring business a year and a half ago, it went great at first but i'm seeking to expand myself more
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a synthesis of the drug sulfathiazole from benzene and any necessaryamine. Sulfathiazole H2N
-
Propose a synthesis of Beginning with cyclohexanone, And 3-bromopropanol? CTI2CTH,CTHhOH
-
Propose a synthesis of 4, 4-dimethyl-2-pentyne (the compound in Problem 14.19) from an alkyl halide and an alkyne.
-
Make a presentation about marketing: Your Companys marketing department promotes the products and interacts with the customers, sales force, and supply chain. They are also in charge of forecasting...
-
1. What do you recommend that Penelope do with the request to form a company-sponsored affinity group? 2. What concerns, if any, do you have about Penelope's commitment to demographic and cultural...
-
An environmental psychologist investigated ways to reduce waste. He randomly assigned office workers in small businesses to three groups: (1) To be in a control group, (2) To receive daily e-mail...
-
Are proper test development procedures used in the design? _
-
What is the traditional accounting focus in managing costs over the total life cycle of a product? What is the problem with this focus?
-
Balance Sheet September 3 0 Assets Cash Accounts receivable 9 0 , 0 0 0 Inventory 3 2 , 4 0 0 Buildings and equipment, net o f depreciation 2 1 4 , 0 0 0 Total assets $ 3 9 5 , 4 0 0 Liabilities and...
-
Mike Wilde is president of the teachers union for Otsego School District. In preparing for upcoming negotiations, he would like to investigate the salary structure of classroom teachers in the...
-
P-Amino benzoic acid (PABA) is widely used as a sunscreen agent. Propose a synthesis of PABA starting from toluene.
-
The pK a ?s of five p-substituted benzoic acids (YC 6 H 4 CO 2 H) follow. Rank the corresponding substituted benzenes (YC 6 H 5 ) in order of their increasing reactivity toward electrophilic aromatic...
-
Consider the following scenario: A firm acquires a strategically related target after successfully fending off four other bidding firms. Under what conditions, if any, can the firm that acquired this...
-
A project requires a $802,000 Initial Investment for equipment. The equipment is estimated to have an eight-year life and a salvage value of $42,000. The project is expected to generate income of...
-
A product has the following costs: $ Per Unit Variable production costs 9.60 Total production costs 15.00 Total variable cost 11.80 Total cost 20.00 22,800 units of the product were manufactured in a...
-
Suppose that Boeing Corporation exported a Boeing 747 to Lufthansa and billed 20 million payable in one year. One-year interest rates are 2% in the United States and 4% in the euro zone. The spot...
-
6. [0/1 Points] DETAILS MY NOTES Find the derivative. f'(x) = f(x) = x9.3x symbolic formatting help
-
1) Explain the following paragraph in your own words. "A nation which has can produce at a lower cost when measured in terms of opportunity cost is said to have a comparative advantage. Even though...
-
WHAT ARE THE STAGES OF BUSINESS PROCESS MANAGEMENT (BPM)?
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Which solid in each pair has the higher melting point and why? a. Fe(s) or CCl4(s) C. Ti(s) or Ne(s) b. KCI(s) or HCl(s) d. HO(s) or HS(s)
-
Mutations can also be caused chemically, and nitrous acid is one of the most potent chemical mutagens. One explanation that has been suggested for the mutagenic effect of nitrous acid is the...
-
Write structural formulas showing how the keto form of uracil (Section 25.2) in mRNA can pair with adenine in DNA through hydrogen bond formation.
-
Predict the approximate geometry in each of the following molecules. water , formaldehyde
-
solve this plz Alba Company is considering the introduction of a new product. To determine the selle price of the product you have The direct material permit The direct labor per unit The variable...
-
Calculate the current ratio collection period for accounts receivable, inventory turnover, gross margin percentage, and return on equity for 2014 and 2015 for the Jordan Corporation. Do not average....
-
A company received $11,000 cash in exchange for 200 shares of the companys common stock. What would the effect of this transaction on the current years accounting equation? Select one: A. No effect...

Study smarter with the SolutionInn App


