Reaction of cyclohexane with mercury (II) acetate in CH3OH rather than H2O, followed by treatment with NaBH4,
Question:
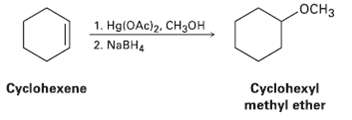
Reaction of cyclohexane with mercury (II) acetate in CH3OH rather than H2O, followed by treatment with NaBH4, yields cyclohexyl methyl ether rather than Cyclohexanol Suggest a mechanism.
Transcribed Image Text:
OCH3 1. Hg(OAc)2, CH3OH 2. NaBH4 Cyclohexyl methyl ether Cyclohexene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
OAc HgOAc HgOAc Cy...View the full answer

Answered By

Pulkit Sahu
1. I have 3 years of tutoring experience in Chemistry subject.
2. 6 years of Industrial Experience in the field of oil and gas.
3. I like to solve problems in Chemistry.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
SimmonsSmith reaction of cyclohexane with di iodomethane gives a single Cyclopropane product, but the analogous reaction of cyclohexane with 1, 1-diiodoethane gives (in low yield) a mixture of two...
-
Methyl acetate (CH3COOCH3) is formed by the reaction of acetic acid with methyl alcohol. If the methyl alcohol is labeled with oxygen-18, the oxygen-18 ends up in the methyl acetate: Do the C-OH bond...
-
A good Williamson synthesis of ethyl methyl ether would be What is wrong with the following proposed synthesis of ethyl methyl ether? First, ethanol is treated with acid to protonate the hydroxyl...
-
Wollongong Group Ltd, of New South Wales, Australia, acquired its factory building about 10 years ago. For several years the company has rented out a small annex attached to the rear of the building....
-
The U.S. Public Interest Research Group Education Fund, USPIRG, recently published a report titled The Campus Credit Card Trap: A Survey of College Students about Credit Card Marketing. You can find...
-
Determine whether the given set, together with the specified operations of addition and scalar multiplication, is a vector space. If it is not, list all of the axioms that fail to hold. The set of...
-
AFN EQUATION Refer to Problem 16-1. What additional funds would be needed if the companys year-end 2014 assets had been $4 million? Assume that all other numbers are the same. Why is this AFN...
-
Analyzing Items to Be Included in Inventory Walker Company has just completed a physical inventory count at year-end, December 31, 2011. Only the items on the shelves, in storage, and in the...
-
b esc lock 86F Sunny Titleist has an advertising slogan, "The #1 ball in golf." Consumers can also buy generic golf balls. The manufacturers of generic golf balls do not engage in any advertising....
-
You are the Owners project manager on an electrical upgrade project. Existing overhead power lines are being buried in a duct bank to hide them from view in an area of the town that is being...
-
Reaction of HBr with 3-methylcyclohexene yields a mixture of four products: cis- and trans-1-bromo-3-methylcyclohexane and cis- and trans-1-bromo- 2-methylcyclohexane. The analogous reaction of HBr...
-
Use your general knowledge of alkene chemistry to suggest a mechanism for the following reaction: Co,CH3 C Hg(OAc)2 AcO-Hg
-
Sapient Corporation is a technology consultancy firm. It focuses on helping clients achieve business outcomes through the rapid application and support of advanced information technology. Most of its...
-
Ranjha Inc. manufactures widgets. The end product is produced in different departments within the plant. One component, C1, is causing some concern. The component is integral to the production of...
-
. Write a Java program in NetBeans that creates a LinkedHashSet. Your Java program must use the methods in the LinkedHashSet interface to do the following: 2.1 Add the above elements into the...
-
on the following statement: Mona is an industrial engineer working for car parts manufacturing facility. She collected the following data on three alternatives of sustainable energy systems to be...
-
Alvarado Company produced 2,900 units of product that required 6 standard direct labor hours per unit. The standard fixed overhead cost per unit is $2.55 per direct labor hour at 16,200 hours, which...
-
Find the complexity of the function given below. void function(int n) { int i, count =0; for(i=1; i*i
-
Consider the model of international settlement rates between the North and the South analyzed in Section 5.3.3. Suppose now that the phone industry in country N is fully competitive, hence the price...
-
The figure shows six containers, each of which is filled from the top. Assume that water is poured into the containers at a constant rate and each container is filled in 10 seconds. Assume also that...
-
The ion CH 5 + can form under very special high-energy conditions in the vapor phase in a mass spectrometer. Propose a hybridization for the carbon atom and predict the geometry.
-
Give a curved- arrow mechanism for the rearrangement shown in {in Eq. 10.12.)
-
Complete the following reactions. If no reaction is tikety, explain why. (a) (b) CH,SH + NaOH -_, (1 equiv.) 25 C CH OH
-
Outline a synthesis of each ether using either alcohol dehydration or alkene addition, as appropriate. (a) 2-methoxy-2-methylbutane (b) dibutyl ether
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...

Study smarter with the SolutionInn App


