Use your general knowledge of alkene chemistry to suggest a mechanism for the following reaction: Co,CH3 C
Question:
Use your general knowledge of alkene chemistry to suggest a mechanism for the following reaction:
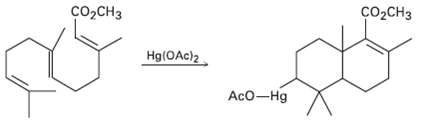
Transcribed Image Text:
Co,CH3 сооCнз Hg(OAc)2 AcO-Hg
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
COCH3 COAC HgOAc ACO Aco COCH3 OAC COCH3 HOC 2 Hq AcO Hg Aco 3 COCH3 COOCH OAc The react...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the following reaction (remember to use curved arrows when showing a mechanism): CH3CHCH-CH-OH CH,C-CH, CH,CHCH-CH-OCCH, + CH CH
-
Provide a mechanism for the following reaction and explain why it occurs faster than nitration of benzene. NO2
-
Propose a mechanism for the following reaction that explains why the configuration of the asymmetric carbon in the reactant is retained in the product: COO NaNO2 HCl NH2 COO
-
Find the probability | (p)| 2d3 p of the particular momentum p for the ground-state hydrogen atom. (This is a nice exercise in three-dimensional Fourier transforms. To perform the angular...
-
A research study showed that adolescents who watched more than 4 hours of TV per day were more than five times as likely to start smoking as those who watched less than 2 hours a day. The researchers...
-
Fill in the values (a) to (f ) in the following table on the assumption that there were no opening balances involved. At end of period Relating to period Accruals/deferred Paid/Received...
-
AFN EQUATION Carter Corporations sales are expected to increase from $5 million in 2014 to $6 million in 2015, or by 20%. Its assets totaled $3 million at the end of 2014. Carter is at full capacity,...
-
Consider a situation in which several tasks may be for one to two years rather than the 200 hours normally used in the work-package level of the WBS. a. How will this affect cost control? b. Can we...
-
Selected data derived from the income statement and balance sheet of National Beverage Co. for a recent year are as follows: 1 Income statement data (in thousands): 2 Net income $49,311.00 3 Gain on...
-
Tiffany Lyons was just hired as the assistant treasurer of Key West Stores. The company is a specialty chain store with nine retail stores concentrated in one metropolitan area. Among other things,...
-
Reaction of cyclohexane with mercury (II) acetate in CH3OH rather than H2O, followed by treatment with NaBH4, yields cyclohexyl methyl ether rather than Cyclohexanol Suggest a mechanism. OCH3 1....
-
Treatment of 4-penten-l-ol with aqueous Br2 yields a cyclic bromo ether rather than the expected bromohydrin. Suggest a mechanism, using curved arrows to show electronmovement. CH2B Br2, H20 %3...
-
Find the efficiency of a cycle consisting of two isochoric and two isothermal lines if the volume varies -fold and the absolute temperature v-fold within the cycle. The working substance is an ideal...
-
analyze the code and fix the error Main.java 2 public class Main 3- { 4 public static void main(String[] args) { 5 6 Scanner myObj-new Scanner(System.in); 7 int count = 0; // sets count to zero CO G...
-
Please provide a reflection on interimsof a C-Level Leader of a start-up technology company, how/what strategy you will consider to grow your business.
-
Using a ruler, completing the following schematics based on the information provided. 19. Draw a wiring diagram of a PSC compressor with a current start relay and a start capacitor.com 20. Draw a...
-
You observe the below command output. *?What is wrong connection timed out; no servers could be reached arya@arya:~$
-
Evaluate your strengths a supervisor and leader using the preferred leadership profile, the key performance motivators scale, the seven domains for inspiration, and other concepts from your course...
-
Answer the previous question assuming that the phone industry in country N remains a monopoly, whereas the phone industry in country S is competitive. Explain the difference between the two scenarios.
-
Establish identity. cos( + k) = (-1)k cos , k any integer
-
The bond angles increase steadily in the series PF 3 , PCl 3 , PBr 3 , and PI 3 . After consulting the data on atomic radii, provide an explanation for this observation.
-
Give the structure of the that would with mCPBA to give each of the following expoxides. (a) (b) . /A C CH2 H,C C-4 CH,
-
The chlorohydrins trans - 2 chlorocyclohexanol reacts rapidly in base to form an epoxide. The cis steroisomer, however, is relatively unreactive and does not give an epoxide. Explain why the two...
-
Explain the following facts with a mechanistic argument. (a) When the reaction mixture in part (a) is heated for times, l-iodobutane is also formed.
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement
-
Entries for equity investments: 20%50% ownership On January 6, 20Y8, Bulldog Co. purchased 25% of the outstanding common stock of $159,000. Gator Co. paid total dividends of $20,700 to all...

Study smarter with the SolutionInn App


