Referring to Exercise 21-C, predict the relative amounts of C 2 H 2 79 Br 2 ,
Question:
Referring to Exercise 21-C, predict the relative amounts of C2H2 79Br2, C2H2 79Br81Br, and C2H2 81Br2 in 1,2-dibromoethylene. Compare your answer with Figure 21-7.
Figure 21-7
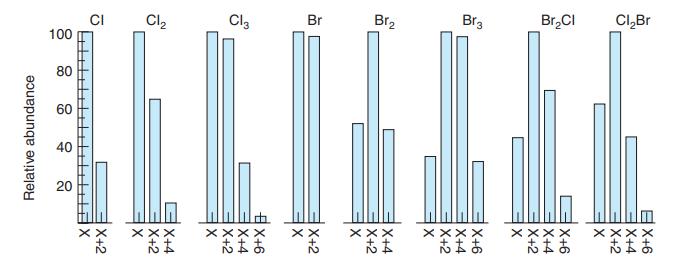
Transcribed Image Text:
CI 100 Cl2 Cl, Br Br2 Br3 Br,CI CI,Br 80 60 40 20 XX く× もまあ XXXX もまあ Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
79 Br abundance 0506 9 8lBrabundance b 04931 Abundanc...View the full answer

Answered By

Ranjan kumar
I Graduated from IIEST Shibpur in 2017 and Currently I am Working in Microsoft for the past 1.7 years. I am a very fun loving and friendly guy who loves to impart knowledge in his free time. I am also associated with Online Tutoring for the past 1 year and have helped more than 1000 Students till now . I gel up with students pretty quickly and train them in the required subjects with utmost care.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
From the natural abundance of 79 Br and 81 Br, predict the relative amounts of CH 79 Br 3 , CH 79 Br 2 81 Br, CH 79 Br 81 Br 2 , and CH 81 Br 3 . As in Exercise 21-C, the fraction of each isotopic...
-
Referring to Exercise 21-C, predict the relative abundances of 10B2H6, 10B11BH6, and 11B2H6 for diborane (B2H6).
-
The temperature-composition diagram for the Ca/Si binary system is shown in Fig. 6.46. (a) Identify eutectics, congruent melting compounds, and incongruent melting compounds. (b) If a 20 per cent by...
-
In the context of supply and demand under international trade, when will a country decide to export a particular good? Import a good? Who gains and loses under each decision?
-
R. Santiago Co. uses special journals and a general journal. The following transactions occurred during May 2014. May 1 R. Santiago invested $40,000 cash in the business. 2 Sold merchandise to Lawrie...
-
Select a value for \(R_{\mathrm{x}}\) so that \(i_{\mathrm{x}}=\mathrm{O}\) A in Figure P2-5 6 . -24 V 60 2 ww Rx ww 20024-0A( 12 V
-
14-7. Si una compaa desea estimular adquisiciones repetidas de un comprador durante un ao, cul estrategia sera mejor, los descuentos por volumen acumulativos o los no acumulativos?
-
HBM, Inc. has the following capital structure: Assets ......$400,000 Debt ....... $140,000 Preferred stock ... 20,000 Common stock .....240,000 The common stock is currently selling for $15 a share,...
-
Of the following statements regarding franchising your own business, which of the following is accurate? Franchisees are more unlikely to leave in the short-term so you can realize a better return...
-
A researcher is interested in assessing the direct and indirect cost of managing electronic health records, design a suitable questionnaire, by including three questions each under the following...
-
Briefly describe how a magnetic sector mass spectrometer works.
-
Find the number of rings plus double bonds in molecules with the following compositions and draw one plausible structure for each: (a) C 11 H 18 N 2 O 3 ; (b) C 12 H 15 BrNPOS; (c) Fragment in a mass...
-
Look up the electron gain enthalpies for Cu, Ag, and Au and the ionization energies of the Group 1 metals. Discuss the likely stability of compounds M + M , where M = Group 1 metal, M = Group 11...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 C. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center....
-
Hello need help with this problem. The transactions relating to the formation of Blue Company Stores Incorporated, and its first month of operations follow. a. The firm was organized and the...
-
At the beginning of the year, the net assets of Shannon Company were $492,600. The only transactions affecting stockholders equity during the year were net income of $70,200 and dividends of $15,400....
-
The claim is that smokers have a mean cotinine level greater than the level of 2.84 ng/mL found for nonsmokers. (Cotinine is used as a biomarker for exposure to nicotine.) The sample size is n = 739...
-
Why is it useful for LEED to incorporate regionalization?
-
Which one of the following anhydrous chloride is not obtained on direct heating of its hydrated chloride? (A) BaCl2 (B) CaClz (C) MgCl2 (D) SrCl2
-
(a) Write the Nernst equations for the half-reactions in Demonstration 14-1. In which direction do electrons move through the circuit? (b) If you use your fingers as a salt bridge in Demonstration...
-
Write a balanced chemical equation (in acidic solution) for the reaction represented by the question mark.22 As in Box 14-5, calculate Eo for the reaction. 1.441 1.491 1.584 1.098 BrO,- HOB - -...
-
What must be the relation between E o+ and E o- if the species X+ is to diproportionate spontaneously under standard conditions to X 3+ and X(s)? Write a balanced equation for the disproportionation.
-
Which of the following statements is true? Financial measures tend to be lag indicators that report on the results of past actions. LA profit center is responsible for generating revenue, but it is...
-
Andretti Company has a single product called a Dak. The company normally produces and sells 8 0 , 0 0 0 Daks each year at a selling price of $ 5 6 per unit. The company s unit costs at this level of...
-
What are the major characteristics of plant assets? Choose one category of PP&E (land, land improvements, buildings or equipment) and describe the costs that may be capitalized with this asset.

Study smarter with the SolutionInn App


