Question: A reversible chemical reaction, A ? B, occurs in the isothermal continuous stirred-tank reactor shown in Fig. E. The rate expressions for the forward and
A reversible chemical reaction, A ? B, occurs in the isothermal continuous stirred-tank reactor shown in Fig. E. The rate expressions for the forward and reverse reactions are: Using the information given below, use a numerical search procedure to determine the value of FB (L/h) that maximizes the production rate of CB (i.e., the amount of CB that leaves the reactor, moles of B per hour). The allowable values of FB are: 0 ? FB ? 200 L/h.Available informationi. The reactor is perfectly mixed.ii. The volume of liquid, V, is maintained constant using an overflow line (not shown in the diagram).iii. The following variables are parameters that are kept constant at the indicated numerical values: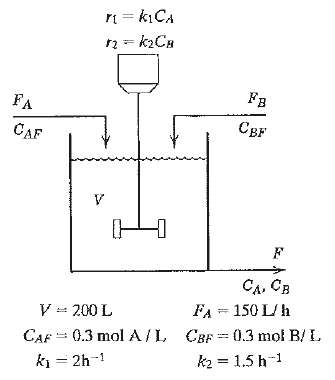
rn = kCA FA CAF CA, CB V = 200 L FA = 150 L/h CBF = 0.3 mol B/L CAF = 0.3 molA/L ki = 2h-1 k2 = 1.5 h-1
Step by Step Solution
3.37 Rating (169 Votes )
There are 3 Steps involved in it
Material balance Thus the optimizati... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
38-E-C-E-P-C (324).docx
120 KBs Word File


