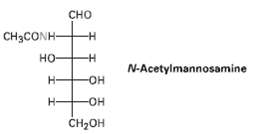
Show how neuraminic acid can arise by an aldol reaction of N-acetylmannosamine with pyruvate (CH 3 COCO
Question:
Show how neuraminic acid can arise by an aldol reaction of N-acetylmannosamine with pyruvate (CH3COCO2?)
Transcribed Image Text:
Cно CH3CONH- но- N-Acetylmannosamine H- OH- Н- -HO- CH-он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
C0 HCH Base HO H H HC 0 0 CH3CONH H ...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
An aldol addition can be catalyzed by acids as well as by bases. Propose a mechanism for the acid-catalyzed aldol addition of propanal.
-
Show how a Wittig reaction can be used to prepare each of the following compounds. In each case, also show how the Wittig reagent would be prepared: (a) (b)
-
Show how you would use an acid chloride as an intermediate to synthesize (a) N-phenylbenzamide (PhCONHPh) from benzoic acid and aniline. (b) Phenyl propionate (CH3CH2COOPh) from propionic acid and...
-
The table below gives the marginal abatement costs of three polluting firms in an industry. The industry is regulated with an emission tax of $9. Emissions. (tons/week) 10 9 8 7 6 5 4 3 2 1 0...
-
How does a synopsis differ from an executive summary?
-
The unadjusted trial balance of Oak and Brass Interiors at December 31, 2012, the end of the current year, is shown below. The data needed to determine year-end adjustments are as follows:a. Supplies...
-
In a small group, select a real organization of any type for study. Review the organizations Web site and/or annual report. For each of the four balanced scorecard areas of performance, identify no...
-
Using the information in E24-3, assume that in July 2010, Raney Company incurs the following manufacturing overhead costs. Instructions(a) Prepare a flexible budget performance report, assuming that...
-
ACCT 1010 W06 Carlie Jensen & 10/20/21 8:22 PM = Homework: Chapter 8 Homework Question 3, E8-22 (book/... Part 1 of 7 HW Score: 40%, 8 of 20 points O Points: 0 of 6 Save Jim Root Company operates...
-
A bag contains 12 identically shaped blocks, 3 of which are red and the remainder are green. The bag is well-shaken and a single block is drawn. a) What is the probability that the block is red? b)...
-
Two of the four D aldopentoses yield D-threose on Wohl degradation. What are their structures?
-
Show the product you would obtain from the reaction of cellobiose with the following reagents: (a) NaBH4 (b) Br2 H2O (c) CH3COCl, pyridine
-
Maurer Corporation had these transactions pertaining to debt investments: Jan. 1 Purchased 90 10%, $1,000 Landis Co. bonds for $90,000 cash. Interest is payable semiannually on July 1 and January 1....
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
Explain the benefits of using the participative approach when leading.
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
A 0.100 M weak acid (HA) solution has a pH of 4.25. Find K a for the acid.
-
What can you say about the stereochemistry of the products in the following reactions? CH-CH2 + H2O CHCH a. - b. CH,CHCH=CH, + H,O CH CH CHCH , (R-enantiomer) OH OH
-
As discussed in the "A Word About . . . Green Chemistry: L-DOPA", the (R,R) DiPAMP phosphine ligand was used to prepare the precursor to L-DOPA. What is the absolute configuration, R or S, of the...
-
Does the staggered conformation of ethane have planes of symmetry? In this conformation, is ethane chiral or achiral?
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


