Show how the Wittig reaction might be used to prepare the following alkenes. Identify the alkyl halide
Question:
Show how the Wittig reaction might be used to prepare the following alkenes. Identify the alkyl halide and the carbonyl components that would he used.
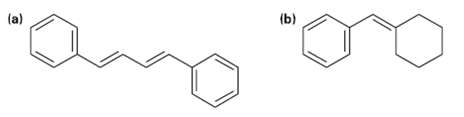
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
Remember RCHX alkyl halide C6H53PCHR X CHCHCHCHL p...View the full answer

Answered By

John Aketch
I have a 10 years tutoring experience and I have helped thousands of students to accomplish their educational endeavors globally. What interests me most is when I see my students being succeeding in their classwork. I am confident that I will bring a great change to thins organization if granted the opportunity. Thanks
5.00+
8+ Reviews
18+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show how a Wittig reaction can be used to prepare each of the following compounds. In each case, also show how the Wittig reagent would be prepared: (a) (b)
-
Show how crossed Claisen condensations could be used to prepare the following esters. (a) (b) (c) (d) Ph C-CH-C OCH,CH CH CH-C-OCH3 C-C-oCH Eto C CH-C OCH,CH Ph CH,CH,CH,
-
Show how a conjugate addition can be used to prepare each of the following compounds. (a) 3,4-dimethyl-2-hexanone (2 ways) (b) levulinic acid
-
Determine the equations for the voltage and instantaneous Power in the network in Figure P 9.2 4 2225 A Figure P 92
-
1. Can you think of additional examples in which diverse organizations have joined forces to address social or environmental problems? 2. Do you believe this co-creation movement is sustainable or a...
-
A political consultant convened a focus group to evaluate the effectiveness of two commercials promoting a candidate. Prior to running any media, the participants are asked to answer one question: Do...
-
Visit an online database or your university library and obtain a copy of a research-based refereed journal article that you think will be of use to an assignment you are currently working on. Read...
-
Swift Manufacturing must choose between two asset purchases. The annual rate of return and the related probabilities given in the following table summarize the firm's analysis to this point. a. For...
-
Great Owl Corp. offers a 7.5 percent coupon bond with annual payments. The yield to maturity is 5 percent and the bond matures 20 years from today. What is the market price of this bond if the face...
-
Spring Manufacturing Company makes two components identified as C12 and D57. Selected budgetary data for 2022 follow: The firm expects the average wage rate to be $25 per hour in 2022. Spring...
-
How would you prepare the following substances from 2-cyclohexcnone? More than one step may be required. .CH (a) (b) (d) (c) CSH5 (Two ways)
-
How would you use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds? (a) 2-Pentanol (b) 1-Butanol (c) 1-Phenylcyclohexanol (d) Diphenyl methanol
-
Decide whether the functions defined as follows are probability density functions on the indicated intervals. If not, tell why. f(x) || x X 21 [1,4]
-
A company which manufactures microwaves advertises that 90% of their microwaves are flawless, requiring no adjustments. Their quality control department tests this percentage on a regular basis. On...
-
A new retail store is being planned for a site that contains 40 ft of soft clay (c 0.075 ft2/day, y = 100 pcf). The clay layer is overlain by 15 ft of sand (y = 112 pcf) and is underlain by dense...
-
Perez Bags (PB) is a designer of high-quality backpacks and purses. Each design is made in small batches. Each spring, PB comes out with new designs for the backpack and for the purse. The company...
-
Find a recent (within the last 12 months) article or economic blog related to price fixing, provide an executive summary of the information. Include an APA reference and/or link. How does the fact...
-
A rectangular block of a material with a modulus of rigidity G=90 ksi is bonded to two rigid horizontal plates. The lower plate is fixed, while the upper plate is subjected to a horizontal force P....
-
Ask the following questions in your internal auditing team and to your customers and stakeholders. Compare the answers and start developing your cutting edge marketing plan. (Reproduced with...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Predict the shapes of the following sulfur-containing species. (a) SO 2 ; (b) SO 3 2- ; (c) SO 4 2 - .
-
Starting with ketones and aldehydes of your choice, outline a directed aldol synthesis of each of the following using lithium enolates: (a) (b) (c) O OH CGH5 O OH
-
Assuming that dehydration occurs, write the structures of the two other products that might have resulted from the aldol cyclization just given. (One of these products will have a five-membered ring...
-
What starting compound would you use in an aldol cyclization to prepare each of the following? (a) (b) (c)
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


